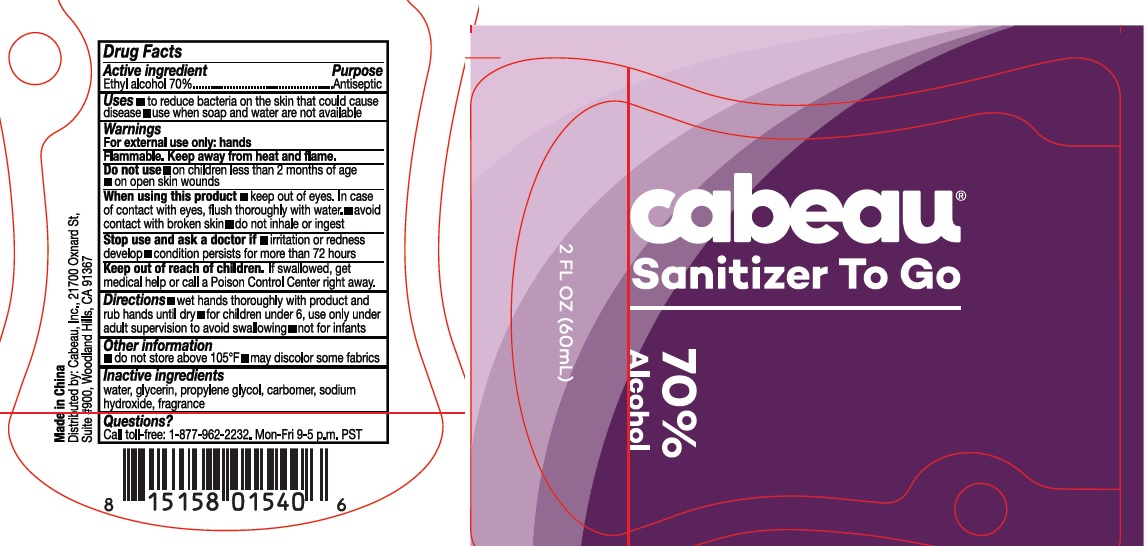
Label: CABEAU SANITIZER TO GO- alcohol gel
- NDC Code(s): 74274-014-01
- Packager: Huizhou Bliss Commodity Co., Ltd
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 7, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Use
- KEEP OUT OF REACH OF CHILDREN
-
warnings:
Flamable, keep away from heat and flames
For external use only: hands
Do not use:
- on children less than 2 months of age
- on open skin wounds
When using this product
- keep out of eyes. In case of contac with eyes, flush thoroughly with water
- avoid contact with broken skin
- do not inhale or ingest
Stop use and ask a doctor if
- Irritation or redness develop
- condition persists for more than 72 hours
- Directions
- Inactive ingredients
- Packaging
-
INGREDIENTS AND APPEARANCE
CABEAU SANITIZER TO GO
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74274-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74274-014-01 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/07/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 05/07/2020 Labeler - Huizhou Bliss Commodity Co., Ltd (417467331) Registrant - Huizhou Bliss Commodity Co., Ltd (417467331) Establishment Name Address ID/FEI Business Operations Huizhou Bliss Commodity Co., Ltd 417467331 manufacture(74274-014)