Label: KOPARI SUN SHIELD SOFT GLOW DAILY FACE SPF30- octisalate, octocrylene, avobenzone, homosalate spray
- NDC Code(s): 68577-153-01
- Packager: COSMAX USA, CORPORATION
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 23, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
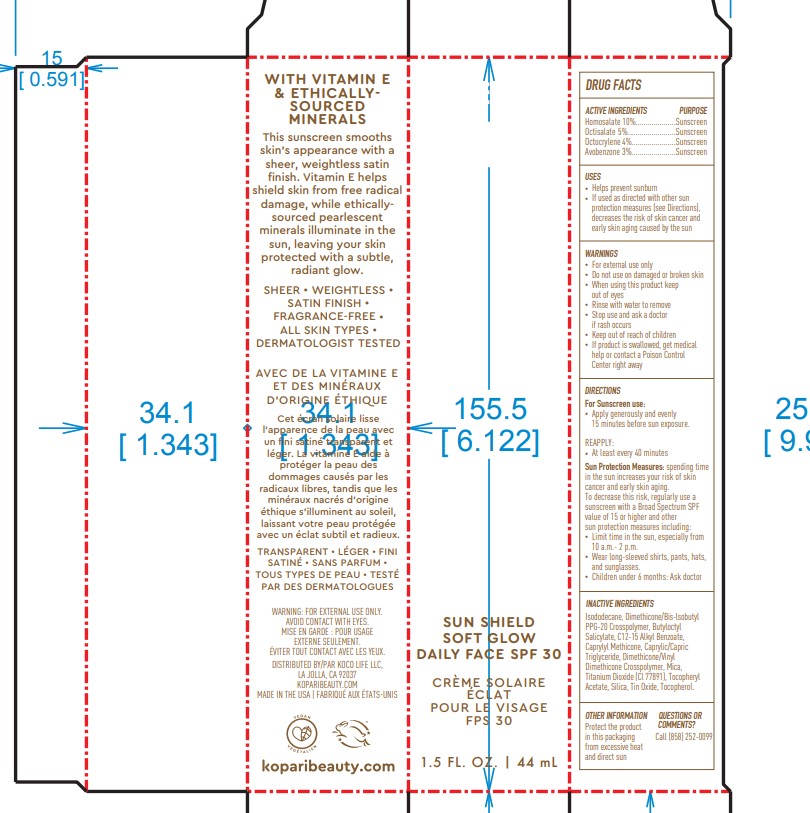
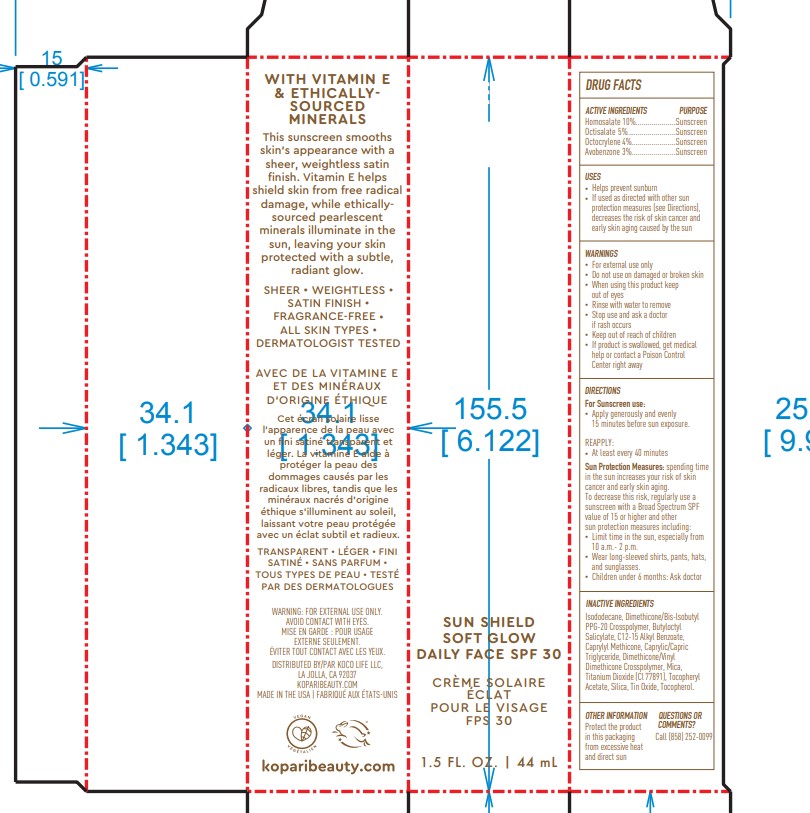
- ACTIVE INGREDIENT
- PURPOSE
- USES
- Warnings
-
DIRECTIONS
Directions
- Apply generously and evenly 15 minutes before sun exposure
- Reapply At least every 40 minutes
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2p.m.
-wear long-sleeved shirts, pants, hats and sunglasses
Children under 6 months of age: Ask a doctor
- Apply generously and evenly 15 minutes before sun exposure
-
INACTIVE INGREDIENT
Inactive ingredients:Isododecane, Dimethicone/Bis-lsobutyl PPG-20 Crosspolymer, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Caprylyl Methicone, Caprylic/Capric Triglyceride, Dimethicone/Vinyl Dimethicone Crosspolymer, Mica, Titanium Dioxide (Cl 77891), Tocopheryl Acetate, Silica, Tin Oxide, Tocopherol.
- OTHER INFORMATION
- QUESTIONS or COMMENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KOPARI SUN SHIELD SOFT GLOW DAILY FACE SPF30
octisalate, octocrylene, avobenzone, homosalate sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68577-153 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 mg in 100 mg HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 mg in 100 mg OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4 mg in 100 mg OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 mg in 100 mg Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STANNOUS OXIDE (UNII: JB2MV9I3LS) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68577-153-01 1 in 1 CARTON 06/01/2023 1 100 mg in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2023 Labeler - COSMAX USA, CORPORATION (010990210) Registrant - COSMAX USA, CORPORATION (010990210) Establishment Name Address ID/FEI Business Operations COSMAX USA. CORPORATION 010990210 manufacture(68577-153)