Label: LIPROTECT SPF 35- zinc oxide, titanium dioxide stick
- NDC Code(s): 69219-112-01, 69219-112-11
- Packager: Science of Skincare
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Apply liberally 15 minutes before sun exposure
Use a water resistance sunscreen if swimming or sweating.
Reapply at least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a board spectrum SPF of 15 or higher and other sun protection measures including: limit time in sun, especially from 10am to 2pm. wear long-sleeve shirts, pants, hats, and sunglasses. Children under the age of 6 months. Ask a doctor.
- OTHER SAFETY INFORMATION
- QUESTIONS
-
INACTIVE INGREDIENT
Vegetable Oil, Cocos Nucifera (Coconut) Oil, Euphorbia Cerifera (Candelilla) Wax, Flavor(Aroma), C12-15 Alkyl Benzoate, Ozokerite, Hydrogenated Vegetable Oil, Sambucus Nigra Fruit Extract, Tocopherol, VP/Eicosene Copolymer, Caprylic/Capric Triglyceride, Dipalmitoyl Hydroxyproline, Simmondsia Chinensis (Jojoba) Seed Oil, Glycine Soja (Soybean) Oil, Polyhydroxystearic Acid, Ethylhexyl Palmitate, Rebaudioside A, Aluminum Stearate, Tribehenin, Palmitoyl Tripeptide-1, Linoleic Acid, Isostearic Acid, Glycine Soja (Soybean) Sterols, Phospholipids, Sorbitan Isostearate, Alumina.
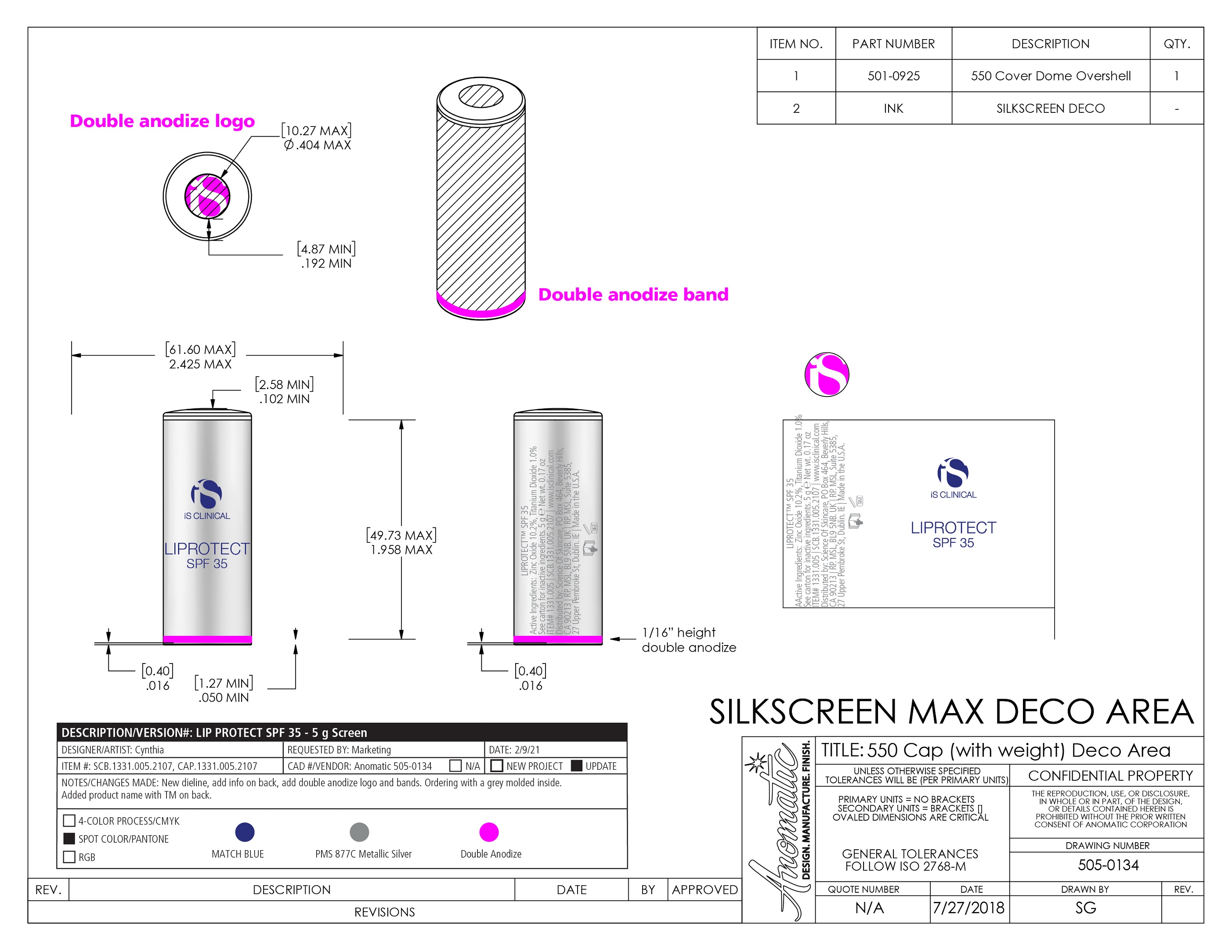
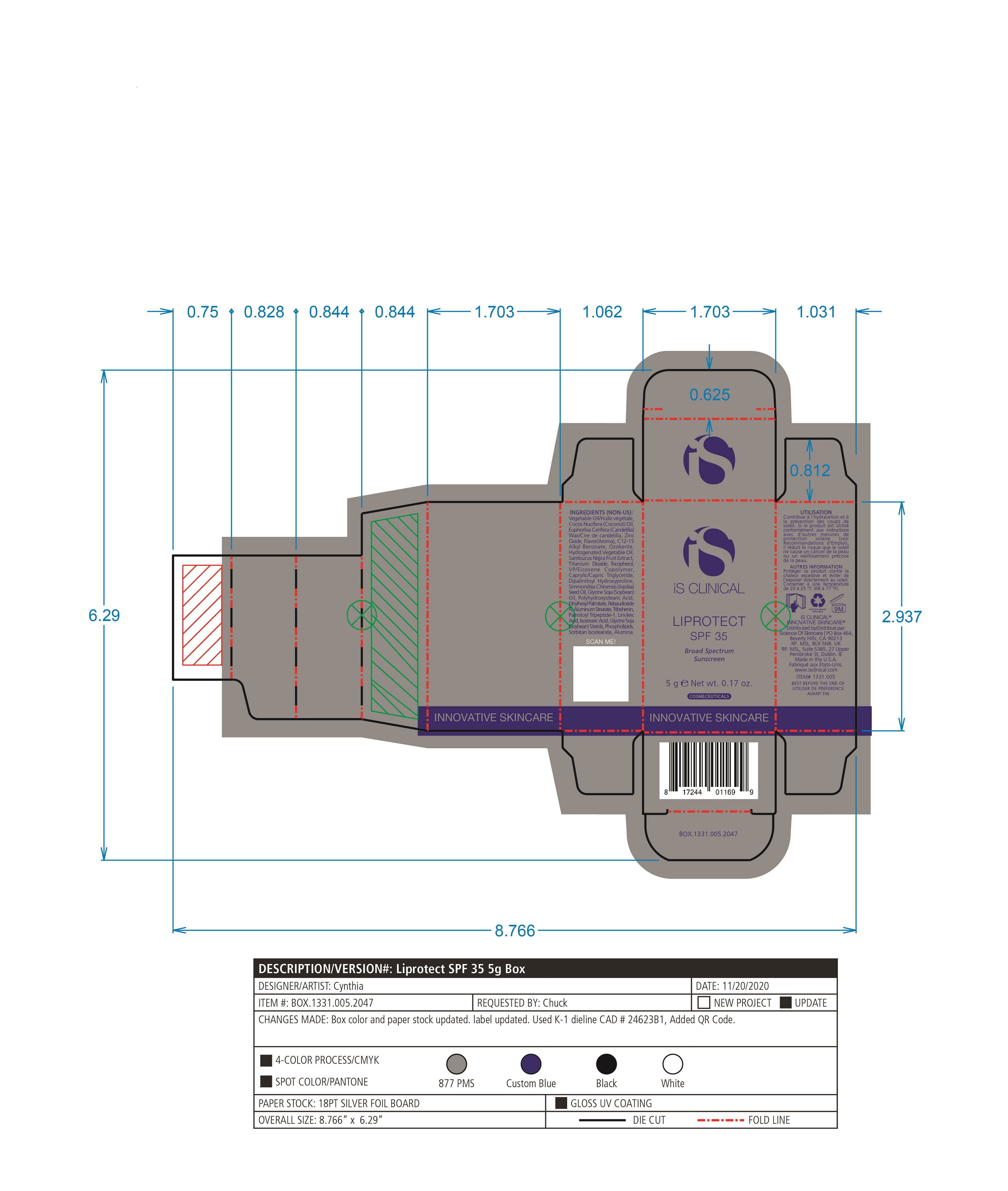
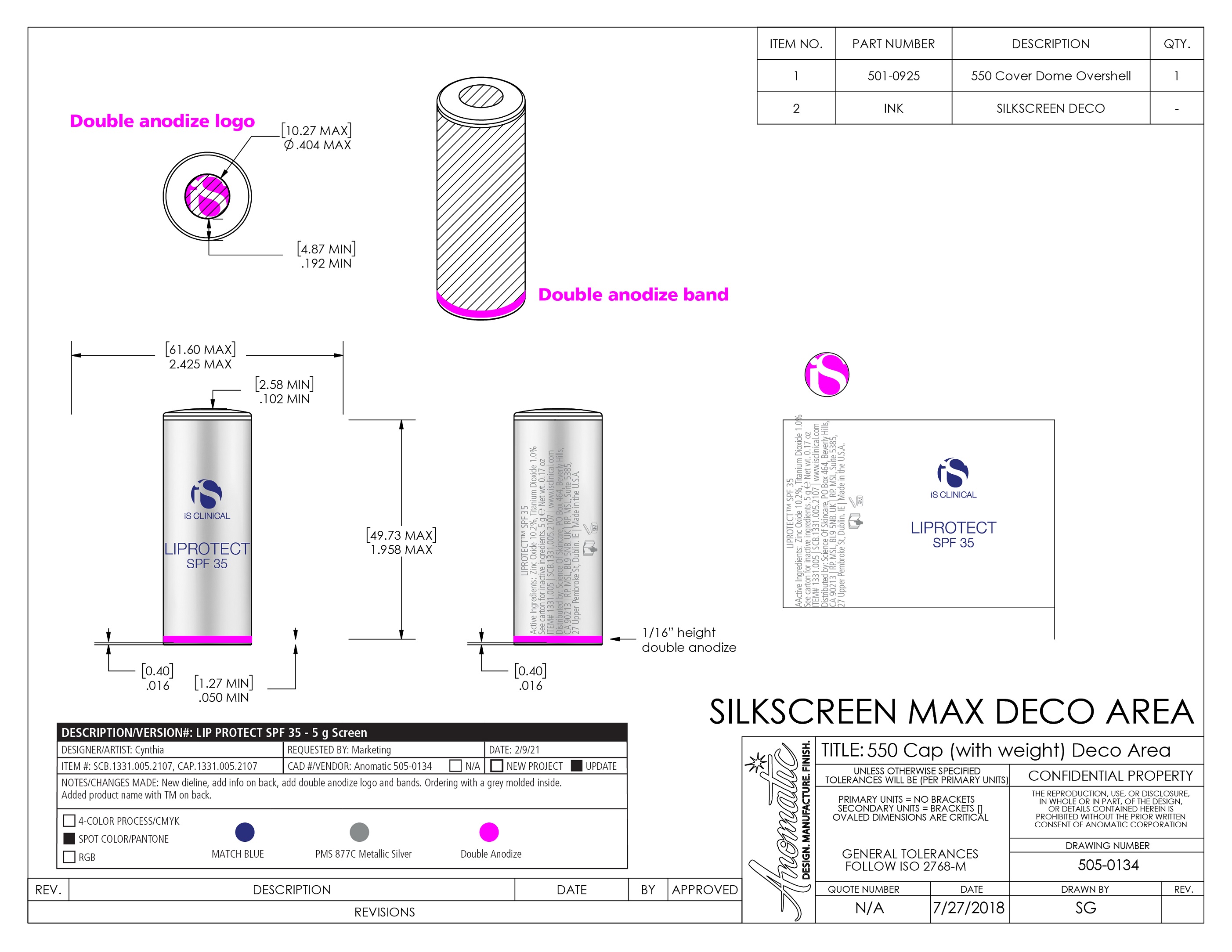
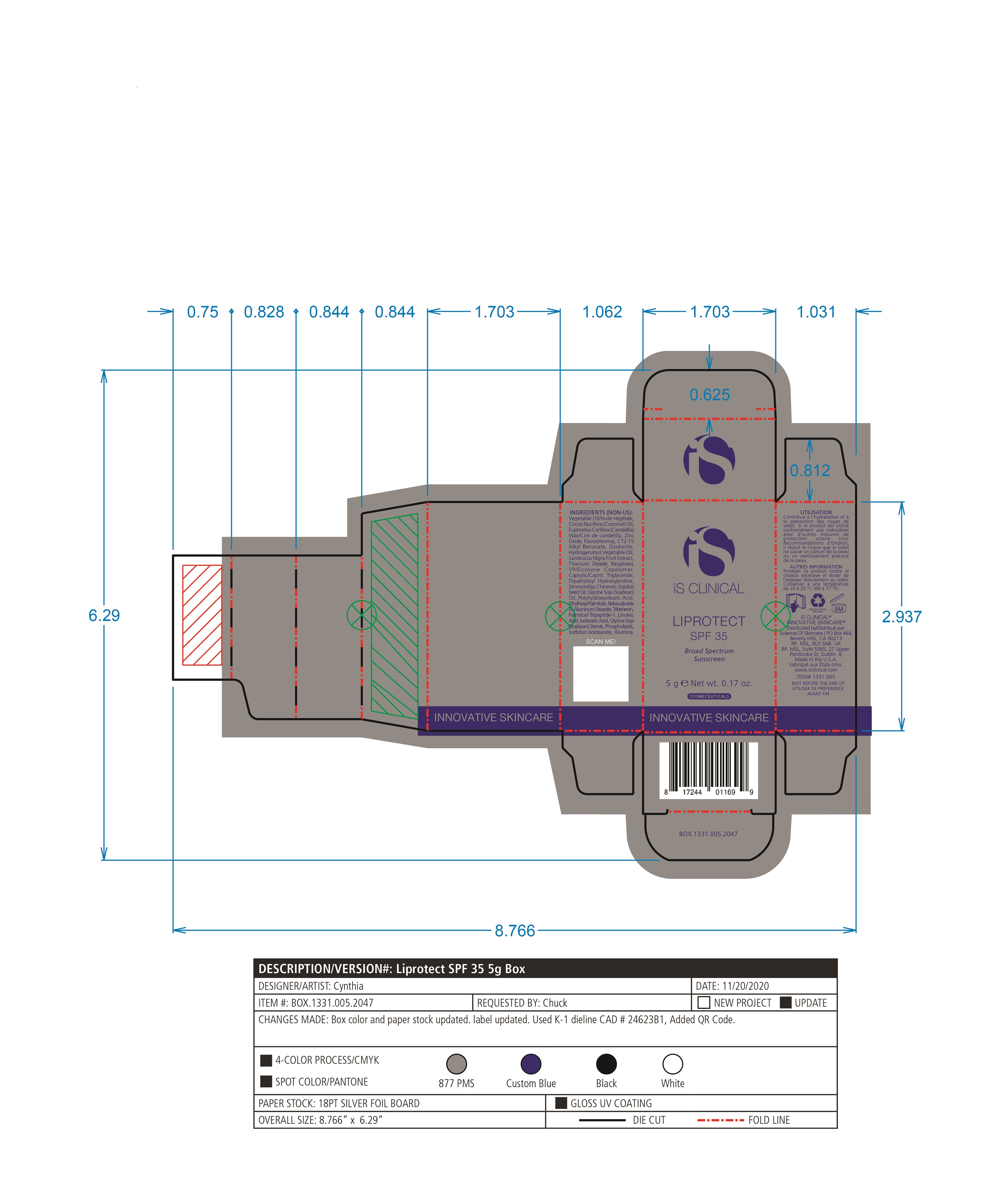
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIPROTECT SPF 35
zinc oxide, titanium dioxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69219-112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 10.2 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1 g in 100 g Inactive Ingredients Ingredient Name Strength REBAUDIOSIDE A (UNII: B3FUD0528F) ISOSTEARIC ACID (UNII: X33R8U0062) SOY STEROL (UNII: PL360EPO9J) ALUMINUM OXIDE (UNII: LMI26O6933) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) LINOLEIC ACID (UNII: 9KJL21T0QJ) EUROPEAN ELDERBERRY (UNII: BQY1UBX046) COCONUT OIL (UNII: Q9L0O73W7L) CANDELILLA WAX (UNII: WL0328HX19) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) TRIBEHENIN (UNII: 8OC9U7TQZ0) SOYBEAN OIL (UNII: 241ATL177A) CERESIN (UNII: Q1LS2UJO3A) CHERRY (UNII: BUC5I9595W) EICOSYL POVIDONE (UNII: XQQ9MKE2BJ) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALUMINUM STEARATE (UNII: U6XF9NP8HM) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) TOCOPHEROL (UNII: R0ZB2556P8) JOJOBA OIL (UNII: 724GKU717M) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) CORN OIL (UNII: 8470G57WFM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69219-112-11 1 in 1 BOX 12/31/2023 1 NDC:69219-112-01 5 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:69219-112-01 5 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/31/2023 Labeler - Science of Skincare (006251958)