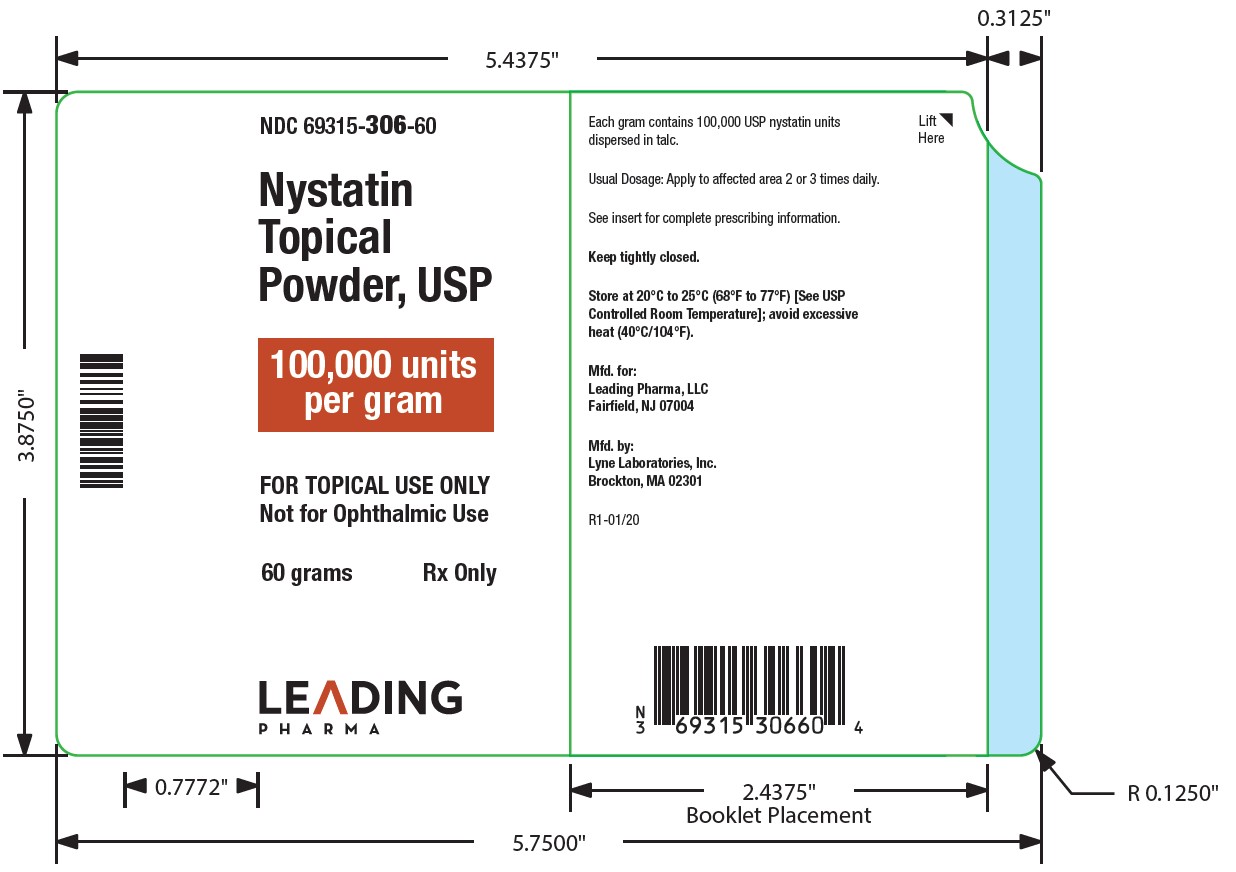
Label: NYSTATIN powder
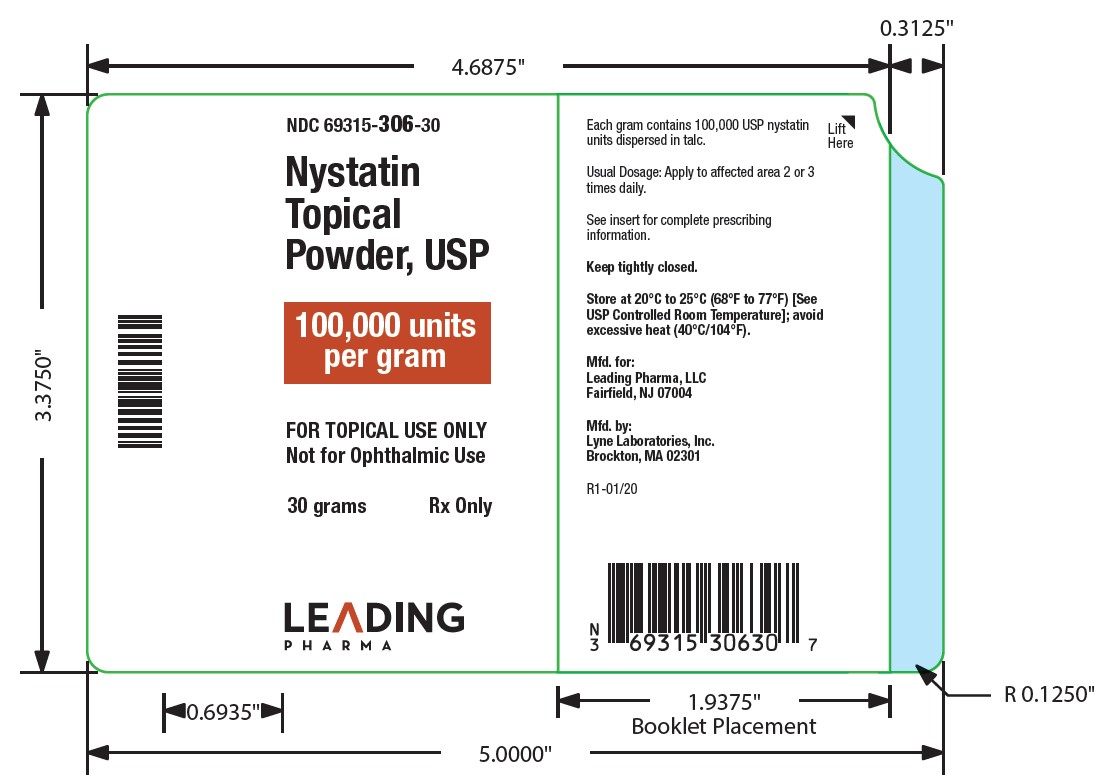
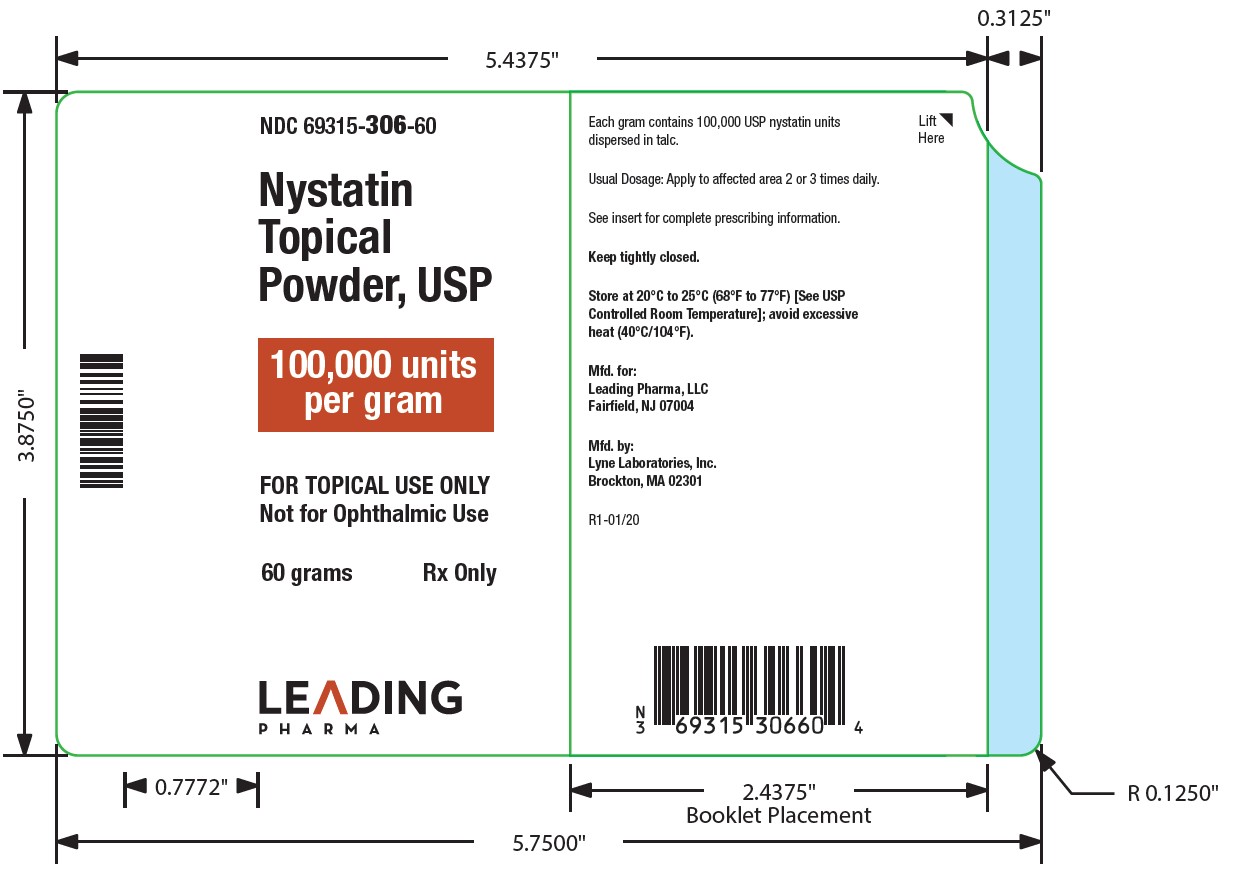
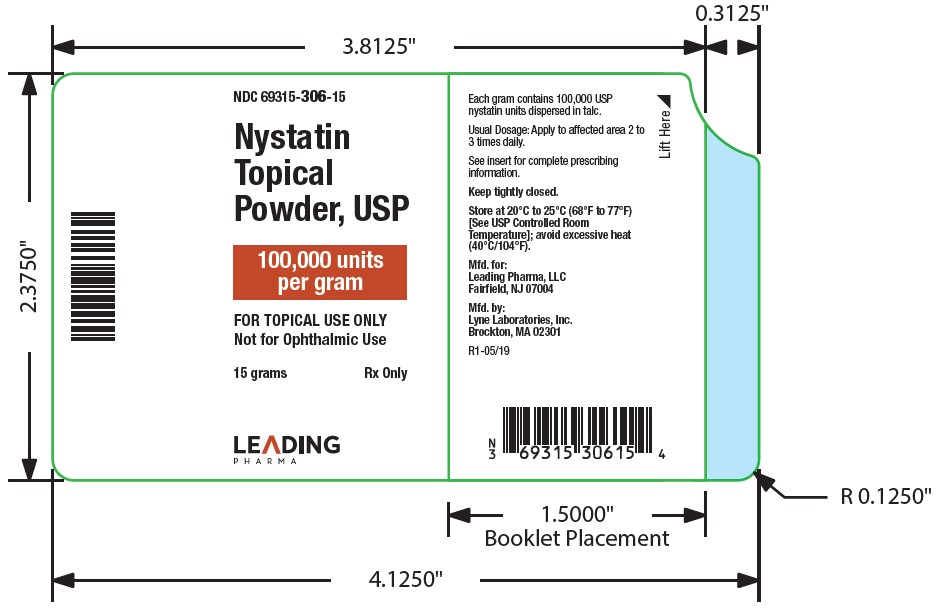
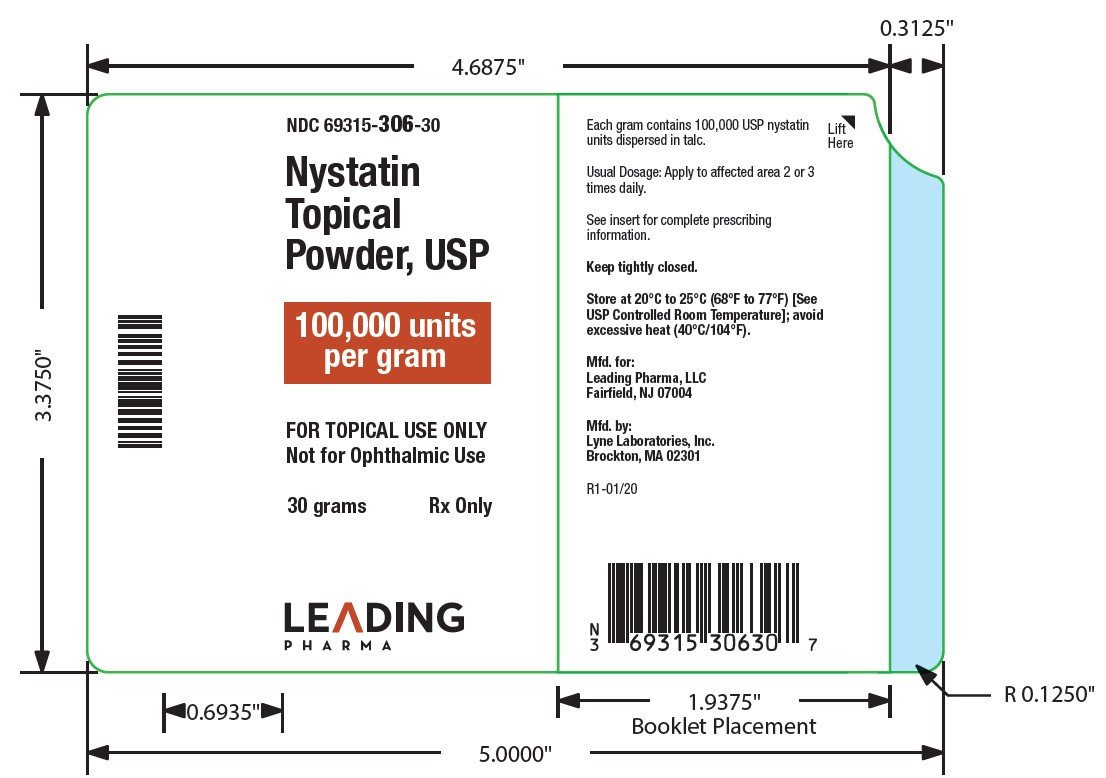
- NDC Code(s): 69315-306-15, 69315-306-30, 69315-306-60
- Packager: Leading Pharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated April 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Nystatin is a polyene antifungal antibiotic obtained from Streptomyces noursei. The molecular formula for Nystatin is C47H75NO17. The molecular weight of Nystatin is 926.1.
Structural formula: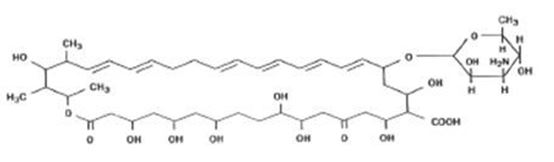
Nystatin topical powder is for dermatologic use.
Nystatin topical powder contains 100,000 nystatin units per gram dispersed in talc. -
CLINCAL PHARMACOLOGY
Pharmacokinetics
Nystatin is not absorbed from intact skin or mucous membrane.
MicrobiologyNystatin is an antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi, including Candida albicans, C. parapsilosis, C. tropicalis, C. guilliermondi, C. pseudotropicalis, C. krusei, Torulopsis glabrata, Tricophyton rubrum, T. mentagrophytes.
Nystatin acts by binding to sterols in the cell membrane of susceptible species resulting in a change in membrane permeability and the subsequent leakage of intracellular components. On repeated subculturing with increasing levels of nystatin, Candida albicans does not develop resistance to nystatin. Generally, resistance to nystatin does not develop during therapy. However, other species of Candida (C. tropicalis, C. guilliermondi, C. krusei, and C. stellatoides) become quite resistant on treatment with nystatin and simultaneously become cross resistant to amphotericin as well. This resistance is lost when the antibiotic is removed.
Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Nystatin topical powder should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections.
If irritation or sensitization develops, treatment should be discontinued and appropriate measures taken as indicated. It is recommended that KOH smears, cultures, or other diagnostic methods be used to confirm the diagnosis of cutaneous or mucocutaneous candidiasis and to rule out infection caused by other pathogens
-
HOW SUPPLIED
Nystatin topical powder is supplied as 100,000 units nystatin per gram in plastic squeeze bottles:
15g (NDC 69315-306-15)30g (NDC 69315-306-30)
60g (NDC 69315-306-60)
STORAGE
Store at 20°C to 25°C (68°F to 77°F)[see USP Controlled Room Temperature]; avoid excessive heat (40°C/104°F).
Keep tightly closed.
Manufactured for:
Leading Pharma LLC, Fairfield, NJ 07004
Manufactured by:
Lyne Laboratories, Inc. Brockton, MA 02301
R1-01/20 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NYSTATIN
nystatin powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69315-306 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NYSTATIN (UNII: BDF1O1C72E) (NYSTATIN - UNII:BDF1O1C72E) NYSTATIN 100000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69315-306-15 15 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/23/2018 2 NDC:69315-306-30 30 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/23/2018 3 NDC:69315-306-60 60 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/23/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208838 04/23/2018 Labeler - Leading Pharma, LLC (079575060) Registrant - Lyne Laboratories, Inc (053510459) Establishment Name Address ID/FEI Business Operations Lyne Laboratories, Inc 053510459 manufacture(69315-306)