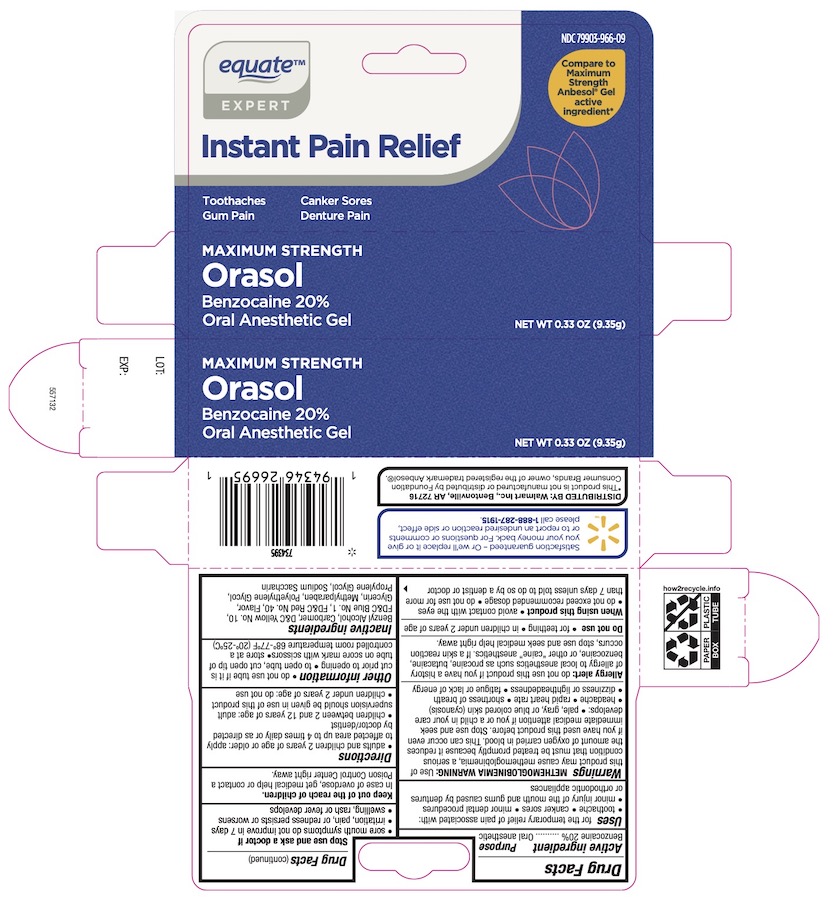
Label: EQUATE ORASOL- 20% benzocaine gel
- NDC Code(s): 79903-966-09
- Packager: WAL-MART STORES INC.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 29, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
-
Warnings
Methemoglobinema Warning: Use of this product may cause Methemoglobinema, a serious condition that must be treated promptly becasue it reduces the amount of oxygem carried in the blood. This can occur even if you have used this product before. Stop use and seek immeditate medical attention if you or a child in yuor care develops;
- pale, gray, or blue colored skin(cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
- Do Not Use
- Stop use and ask a doctor if
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- do not use tube if it is cut prior to opening
- cut open tip of tube on score mark
- use your fingertip or cotton applicator to apply a small pea-size amount of Oral Pain Relief Gel
- apply to affected area up to four times daily or as directed by a dentist or physician
- Adults and children 2 years of age and older: Apply to affected area
- Children under 12 years of age should be supervised in the use of this product
- Children under 2 years of age: Consult a doctor
- Inactive ingredients
- Other information
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
EQUATE ORASOL
20% benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-966 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER 940 (UNII: 4Q93RCW27E) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-966-09 1 in 1 CARTON 04/30/2024 1 9.35 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 04/30/2024 Labeler - WAL-MART STORES INC. (051957769)