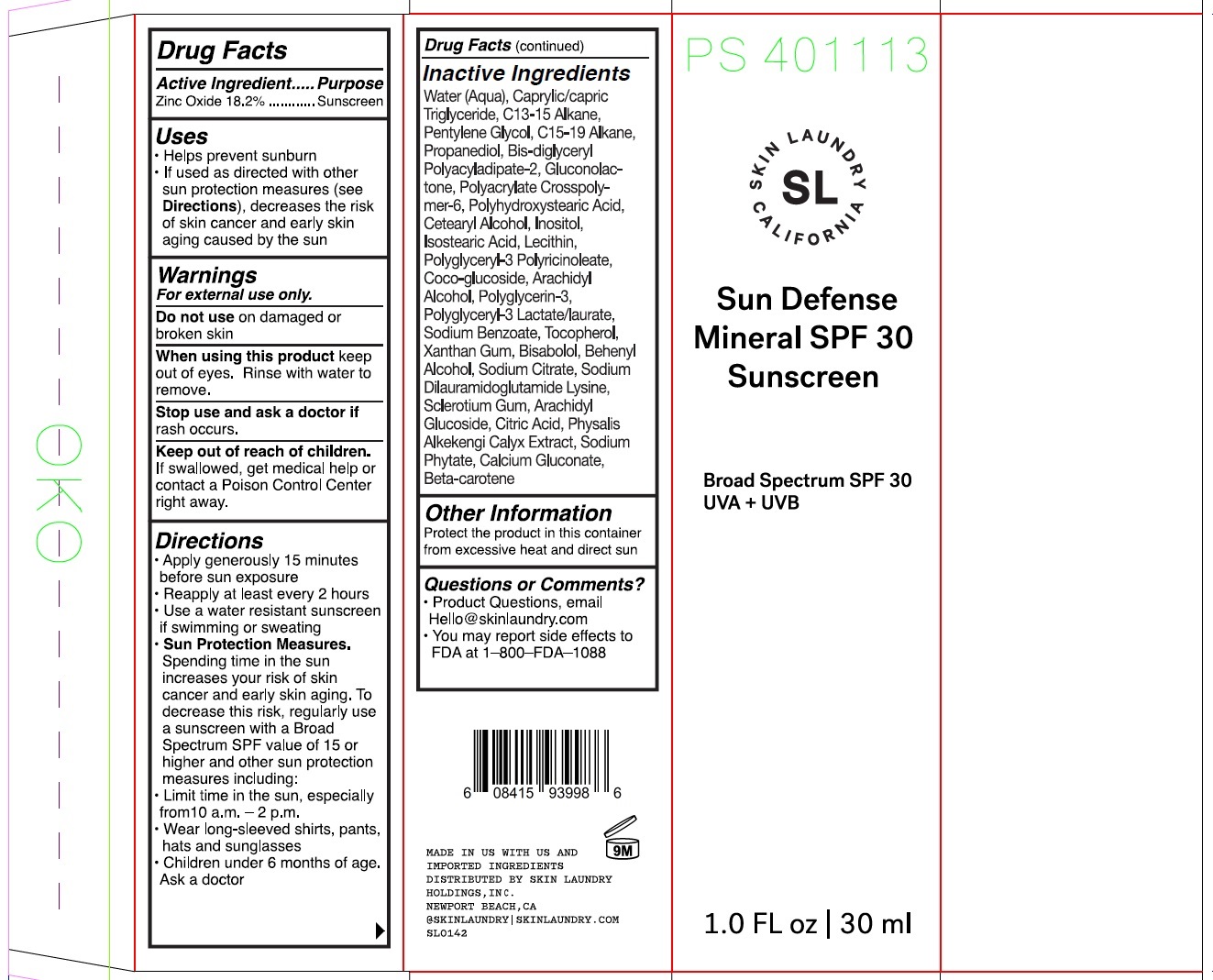
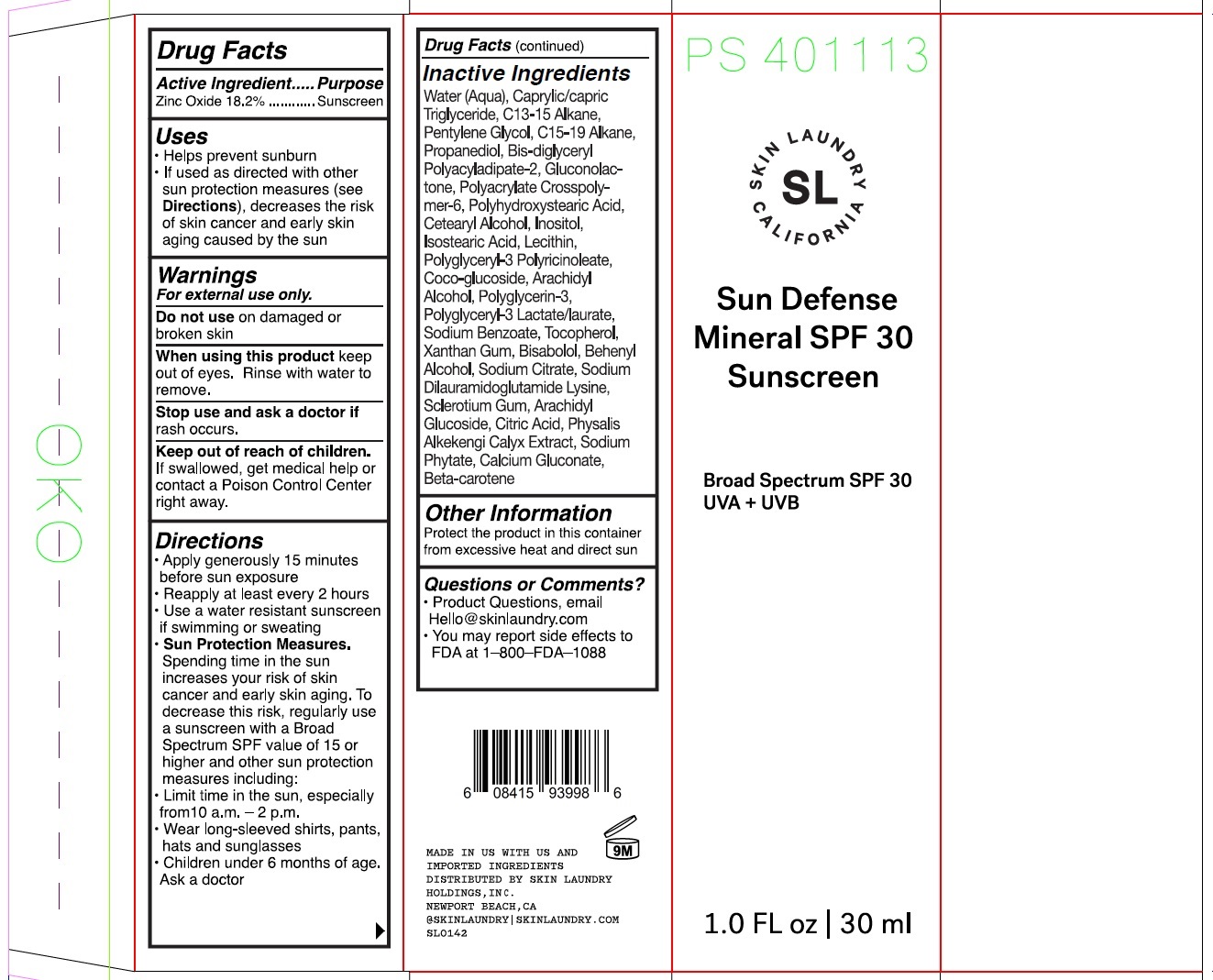
Label: SKIN LAUNDRY SUN DEFENSE MINERAL SPF 30 SUNSCREEN BROAD SPECTRUM SPF 30 UVA UVB- zinc oxide cream
- NDC Code(s): 83727-295-00
- Packager: Skin Laundry Holdings, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 6, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
-
Directions
● Apply generously 15 minutes before sun exposure ● Reapply at least every 2 hours ● Use a water resistant sunscreen if swimming or sweating ● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin ging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: ● Limit time in the sun, especially from 10 a.m. - 2 p.m. ● Wear long-sleeved shirts, pants, hats, and sunglasses ● Children under 6 months: Ask a doctor
-
Inactive ingredients
Water (Aqua), Caprylic/Capric Triglyceride, C13-15 Alkane, Pentylene Glycol, C15-19 Alkane, Propanediol, Bis-Diglyceryl Polyacyladipate-2, Gluconolactone, Polyacrylate Crosspolymer-6, Polyhydroxystearic Acid, Cetearyl Alcohol, Inositol, Isostearic Acid, Lecithin, Polyglyceryl-3 Polyricinoleate, Coco-Glucoside, Arachidyl Alcohol, Polyglycerin-3, Polyglyceryl-3 Lactate/Laurate, Sodium Benzoate, Tocopherol, Xanthan Gum, Bisabolol, Behenyl Alcohol, Sodium Citrate, Sodium Dilauramidoglutamide Lysine, Sclerotium Gum, Arachidyl Glucoside, Citric Acid, Physalis Alkekengi Calyx Extract, Sodium Phytate, Calcium Gluconate, Beta-Carotene
- Other information
- Question or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SKIN LAUNDRY SUN DEFENSE MINERAL SPF 30 SUNSCREEN BROAD SPECTRUM SPF 30 UVA UVB
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83727-295 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 182 mg in 1 mL Inactive Ingredients Ingredient Name Strength ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ALKEKENGI OFFICINARUM CALYX (UNII: AL7F9NO9HR) PHYTATE SODIUM (UNII: 88496G1ERL) CALCIUM GLUCONATE (UNII: SQE6VB453K) BETA CAROTENE (UNII: 01YAE03M7J) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) SODIUM BENZOATE (UNII: OJ245FE5EU) TOCOPHEROL (UNII: R0ZB2556P8) XANTHAN GUM (UNII: TTV12P4NEE) LEVOMENOL (UNII: 24WE03BX2T) DOCOSANOL (UNII: 9G1OE216XY) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) C13-15 ALKANE (UNII: 114P5I43UJ) PENTYLENE GLYCOL (UNII: 50C1307PZG) C15-19 ALKANE (UNII: CI87N1IM01) PROPANEDIOL (UNII: 5965N8W85T) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) GLUCONOLACTONE (UNII: WQ29KQ9POT) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INOSITOL (UNII: 4L6452S749) ISOSTEARIC ACID (UNII: X33R8U0062) COCO GLUCOSIDE (UNII: ICS790225B) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) BETASIZOFIRAN (UNII: 2X51AD1X3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83727-295-00 1 in 1 CARTON 10/14/2023 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/14/2023 Labeler - Skin Laundry Holdings, Inc. (118584348)