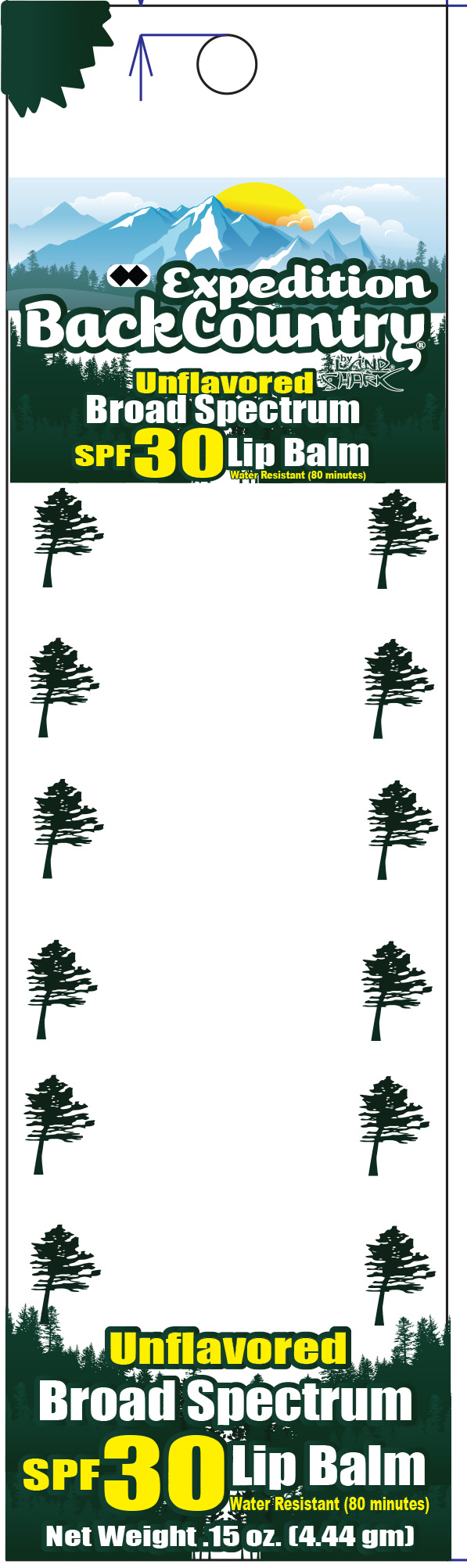
Label: EXPEDITION BACKCOUNTRY SPF 30- octocrylene, octisalate, avobenzone lipstick
- NDC Code(s): 52854-976-01
- Packager: Tropical Seas, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 3, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
ricinus communis (castor) seed oil, cetearyl alcohol, lauryl laurate, olea europaea (olive) fruit oil, hydrogenated castor oil, beeswax, copernicia cerifera (carnauba) wax, flavor, hydrogenated soybean oil, euphorbia cerifera (candelilla) wax, flavor, glyceryl stearate, octyl dodecyl citrate crosspolymer, phenethyl benzoate, cocos nucifera (coconut) fruit extract, octyldodecyl neopentanoate, cetyl dimethicone, hippophae rhamnoides (sea buckthorn) oil.
- Principal Display Panel - Label
-
INGREDIENTS AND APPEARANCE
EXPEDITION BACKCOUNTRY SPF 30
octocrylene, octisalate, avobenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52854-976 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 3 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.5 g in 100 g Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAURYL LAURATE (UNII: GPW77G0937) OLIVE OIL (UNII: 6UYK2W1W1E) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) YELLOW WAX (UNII: 2ZA36H0S2V) CARNAUBA WAX (UNII: R12CBM0EIZ) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) CANDELILLA WAX (UNII: WL0328HX19) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PHENETHYL BENZOATE (UNII: 0C143929GK) COCONUT (UNII: 3RT3536DHY) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) CETYL DIMETHICONE 150 (UNII: 5L694Y0T22) HIPPOPHAE RHAMNOIDES SEED OIL (UNII: T53SBG6741) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52854-976-01 4.44 g in 1 TUBE; Type 0: Not a Combination Product 08/10/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/10/2023 Labeler - Tropical Seas, Inc. (627865660) Establishment Name Address ID/FEI Business Operations Tropical Seas, Inc. 627865660 manufacture(52854-976)