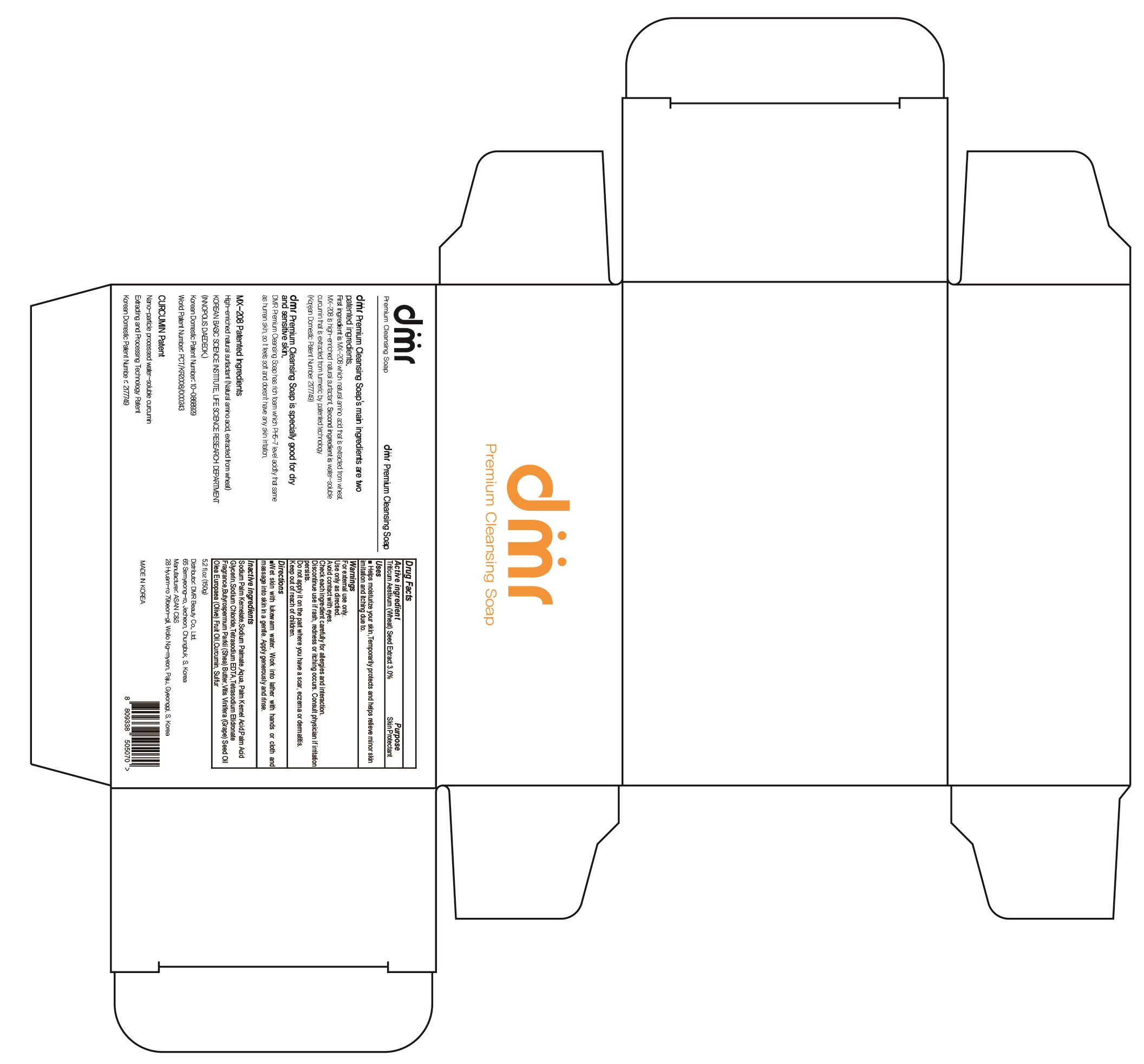
Label: DMR PREMIUM CLEANSING- wheat soap
-
Contains inactivated NDC Code(s)
NDC Code(s): 82629-010-01, 82629-010-02, 82629-010-03 - Packager: DMR Beauty Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 31, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENTS
- PURPOSE
-
WARNINGS
For external use only.
Use only as directied.
Avoid contact with eyes.
Check each ingredient carefully for allergies and interaction.
Discontinue use if rash, redness or itching occurs. Consult physician if irritation persists.
Do not apply it on the part where you have a scar, eczema or dermatitis.
Keep out of reach of children. - KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- 82629-010-01 : DMR Premium Cleansing Soap 150g (1ea)
- 82629-010-03 : DMR Premium Cleansing Soap 150g (3ea)
-
INGREDIENTS AND APPEARANCE
DMR PREMIUM CLEANSING
wheat soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82629-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WHEAT (UNII: 4J2I0SN84Y) (WHEAT - UNII:4J2I0SN84Y) WHEAT 3.0 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM PALM KERNELATE (UNII: 6H91L1NXTW) Sodium Palmate (UNII: S0A6004K3Z) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82629-010-01 150 g in 1 CARTON; Type 0: Not a Combination Product 04/01/2022 2 NDC:82629-010-03 3 in 1 BOX 04/01/2022 2 NDC:82629-010-02 150 g in 1 CARTON; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/01/2022 Labeler - DMR Beauty Co., Ltd. (695422149) Registrant - DMR Beauty Co., Ltd. (695422149) Establishment Name Address ID/FEI Business Operations ASAN C&S 631139649 manufacture(82629-010)