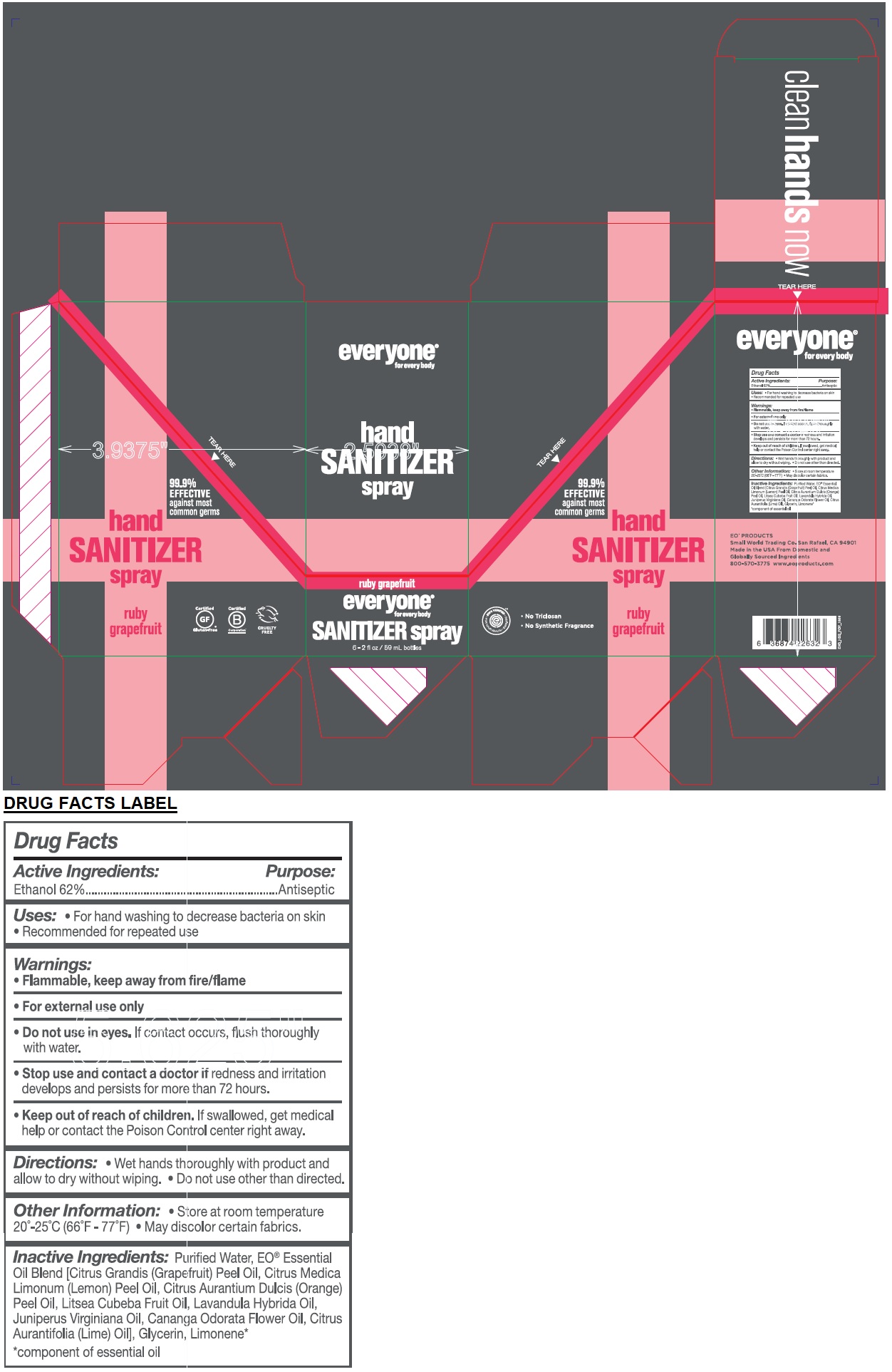
Label: EVERYONE HAND SANITIZER RUBY GRAPEFRUIT- alcohol spray
- NDC Code(s): 54748-304-05, 54748-304-08, 54748-304-09, 54748-304-13
- Packager: EO Products, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 14, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients:
- Purpose:
- INDICATIONS & USAGE
- Warnings:
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
Inactive Ingredients:
Water, Citrus Grandis (Grapefruit) Peel Oil, Citrus Medica Limonum (Lemon) Peel Oil, Citrus Aurantium Dulcis (Orange) Peel Oil, Litsea Cubeba Fruit Oil, Lavandula Hybrida Oil, Juniperus Virginiana Oil, Cananga Odorata Flower Oil, Citrus Aurantifolia (Lime) Oil], Glycerin, Limonene*
*component of essential oil
-
SPL UNCLASSIFIED SECTION
for everybody
99.9%
EFFECTIVE
against most
common germsruby grapefruit
no thymol | no benzalkonium chloride | no triclosan
EO® PRODUCTS
Small World Trading Co.
San Rafael, CA 94901
Made in the USA From Domestic and
Globally Sourced Components
800-570-3775Follow us: everyoneproducts
eoproducts.com
made with moisturizing ingredients
HDPE
PLEASE RECYCLE
Certified Corporation
Certified Gluten-Free
CRUELTY FREE
EWG VERIFIED
FOR YOUR HEALTH
EWG.ORG
- Packaging
-
INGREDIENTS AND APPEARANCE
EVERYONE HAND SANITIZER RUBY GRAPEFRUIT
alcohol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54748-304 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CITRUS MAXIMA FRUIT RIND OIL (UNII: 8U3877WD44) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) ORANGE OIL, COLD PRESSED (UNII: AKN3KSD11B) LITSEA OIL (UNII: 2XIW34BN6O) LAVANDIN OIL (UNII: 9RES347CKG) JUNIPERUS VIRGINIANA OIL (UNII: PAD4FN7P2G) YLANG-YLANG OIL (UNII: 8YOY78GNNX) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) GLYCERIN (UNII: PDC6A3C0OX) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54748-304-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2020 2 NDC:54748-304-09 6 in 1 BOX 09/01/2020 2 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:54748-304-05 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/03/2022 4 NDC:54748-304-13 1 in 1 BOX 02/03/2022 4 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/01/2020 Labeler - EO Products, LLC (786611210) Establishment Name Address ID/FEI Business Operations EO Products, LLC 786611210 manufacture(54748-304)