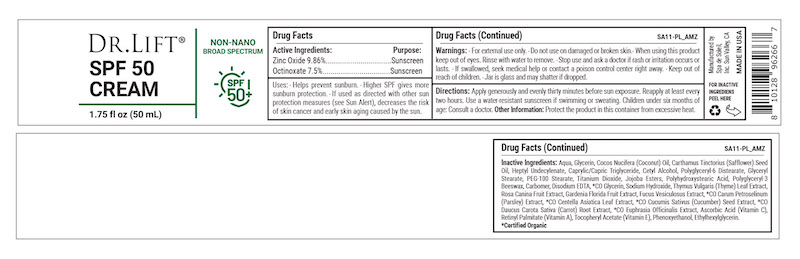
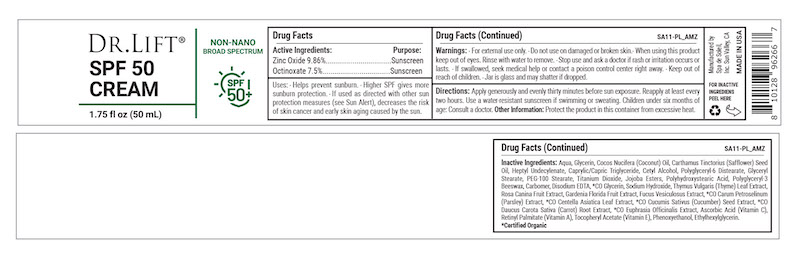
Label: DR LIFT SPF 50 CREAM- titanium dioxide, zinc oxide cream
- NDC Code(s): 68062-9007-1
- Packager: Spa de Soleil
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 24, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- INDICATIONS & USAGE
-
Inactive Ingredients
Inactive Ingredients: Aqua, Glycerin, Cocos Nucifera (Coconut) Oil, Carthamus Tinctorius (Safflower) Seed Oil, Heptyl Undecylenate, Caprylic/Capric Triglyceride, Cetyl Alcohol, Polyglyceryl-6 Distearate, Glyceryl Stearate, PEG-100 Stearate, Titanium Dioxide, Jojoba Esters, Polyhydroxystearic Acid, Polyglyceryl-3 Beeswax, Carbomer, Disodium EDTA, *CO Glycerin, Sodium Hydroxide, Thymus Vulgaris (Thyme) Leaf Extract, Rosa Canina Fruit Extract, Gardenia Florida Fruit Extract, Fucus Vesiculosus Extract, *CO Carum Petroselinum (Parsley) Extract, *CO Centella Asiatica Leaf Extract, *CO Cucumis Sativus (Cucumber) Seed Extract, *CO Daucus Carota Sativa (Carrot) Root Extract, *CO Euphrasia Officinalis Extract, Ascorbic Acid (Vitamin C), Retinyl Palmitate (Vitamin A), Tocopheryl Acetate (Vitamin E), Phenoxyethanol, Ethylhexylglycerin.
*Certified Organic
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DR LIFT SPF 50 CREAM
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68062-9007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 4.93 mg in 50 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.75 mg in 50 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HEPTYL UNDECYLENATE (UNII: W77QUB6GXO) SAFFLOWER OIL (UNII: 65UEH262IS) GLYCERIN (UNII: PDC6A3C0OX) COCONUT OIL (UNII: Q9L0O73W7L) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68062-9007-1 50 mL in 1 TUBE; Type 0: Not a Combination Product 08/24/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 08/24/2023 Labeler - Spa de Soleil (874682867) Establishment Name Address ID/FEI Business Operations Spa de Soleil 874682867 manufacture(68062-9007)