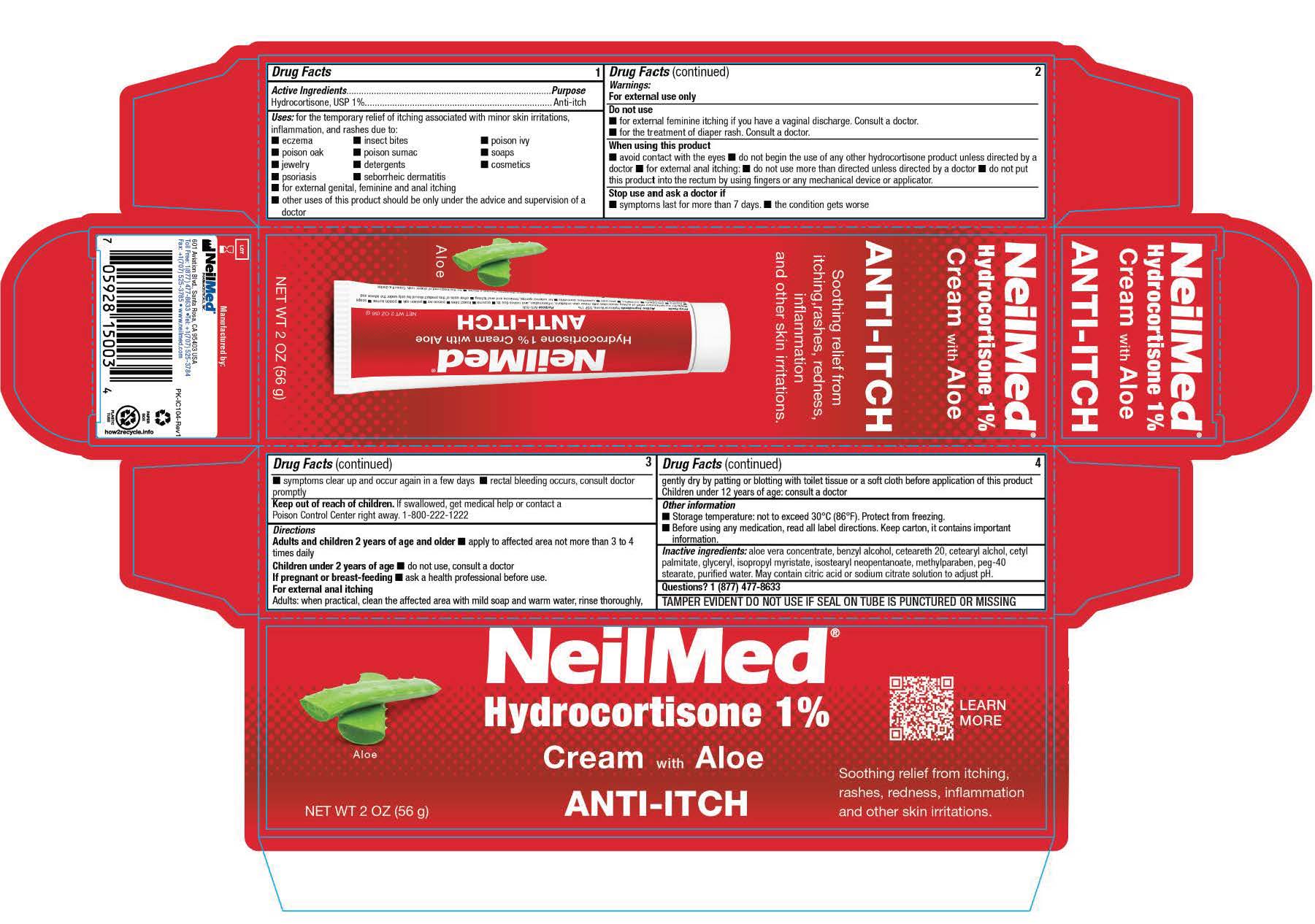
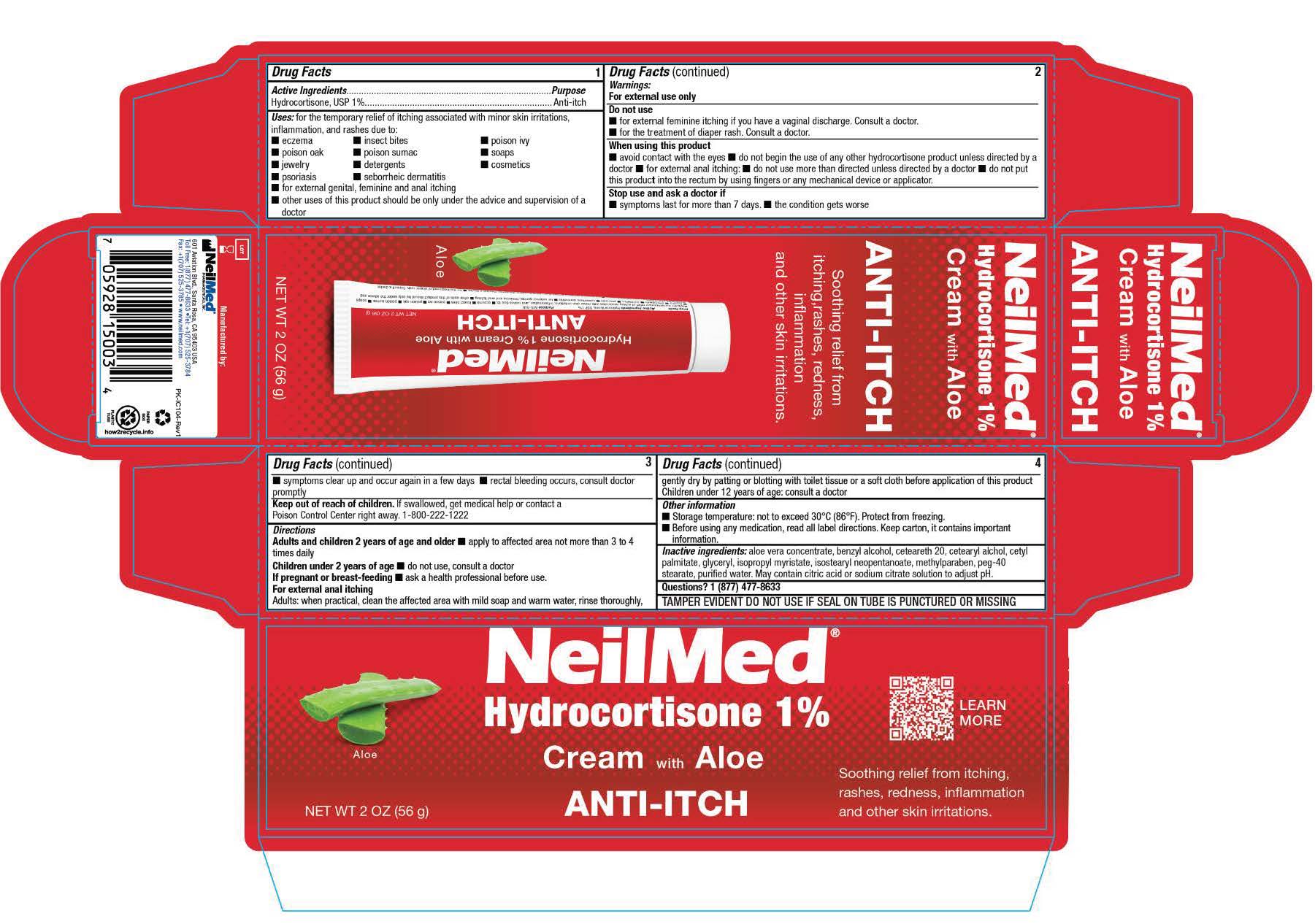
Label: HYDROCORTISONE WITH ALOE- hydrocortisone with aloe anti itch cream
- NDC Code(s): 13709-317-01, 13709-317-02
- Packager: NeilMed Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 26, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
-
Uses:
for the temporary relief of itching associated with minor skin irritations, inflammation, and rashes due to: ■ eczema ■ insect bites ■ poison ivy ■ poison oak ■ poison sumac ■ soaps
■ jewelry ■ detergents ■ cosmetics ■ psoriasis ■ seborrheic dermatitis ■ for external genital, feminine and anal itching ■ other uses of this product should be only under the advice and
supervision of a doctor - Warnings:
-
When using this product
■ avoid contact with the eyes ■ do not begin the use of any other hydrocortisone product unless directed by a doctor ■ for external anal itching: ■ do not use more than directed unless directed by a doctor ■ do not put this product into the rectum by using fingers or any mechanical device or applicator.
- Stop use and ask a doctor if
- Keep out of reach of children
-
Directions
Adults and children 2 years of age and older ■ apply to affected area not more than 3 to 4 times daily
Children under 2 years of age ■ do not use, consult a doctor If pregnant or breast-feeding ■ ask a health professional before use.
For external anal itching Adults: when practical, clean the affected area with mild soap and warm water, rinse thoroughly, gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product Children under 12 years of age: consult a doctor - Other information:
- Inactive ingredients:
- SAFE HANDLING WARNING
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE WITH ALOE
hydrocortisone with aloe anti itch creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13709-317 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10.2 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) BENZYL ALCOHOL (UNII: LKG8494WBH) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) PEG-40 MONOSTEARATE (UNII: ECU18C66Q7) CETYL PALMITATE (UNII: 5ZA2S6B08X) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13709-317-01 1 in 1 CARTON 08/18/2023 1 28 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:13709-317-02 1 in 1 CARTON 08/18/2023 2 56 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 08/18/2023 Labeler - NeilMed Pharmaceuticals Inc. (799295915) Establishment Name Address ID/FEI Business Operations NeilMed Pharmaceuticals Inc. 799295915 manufacture(13709-317)