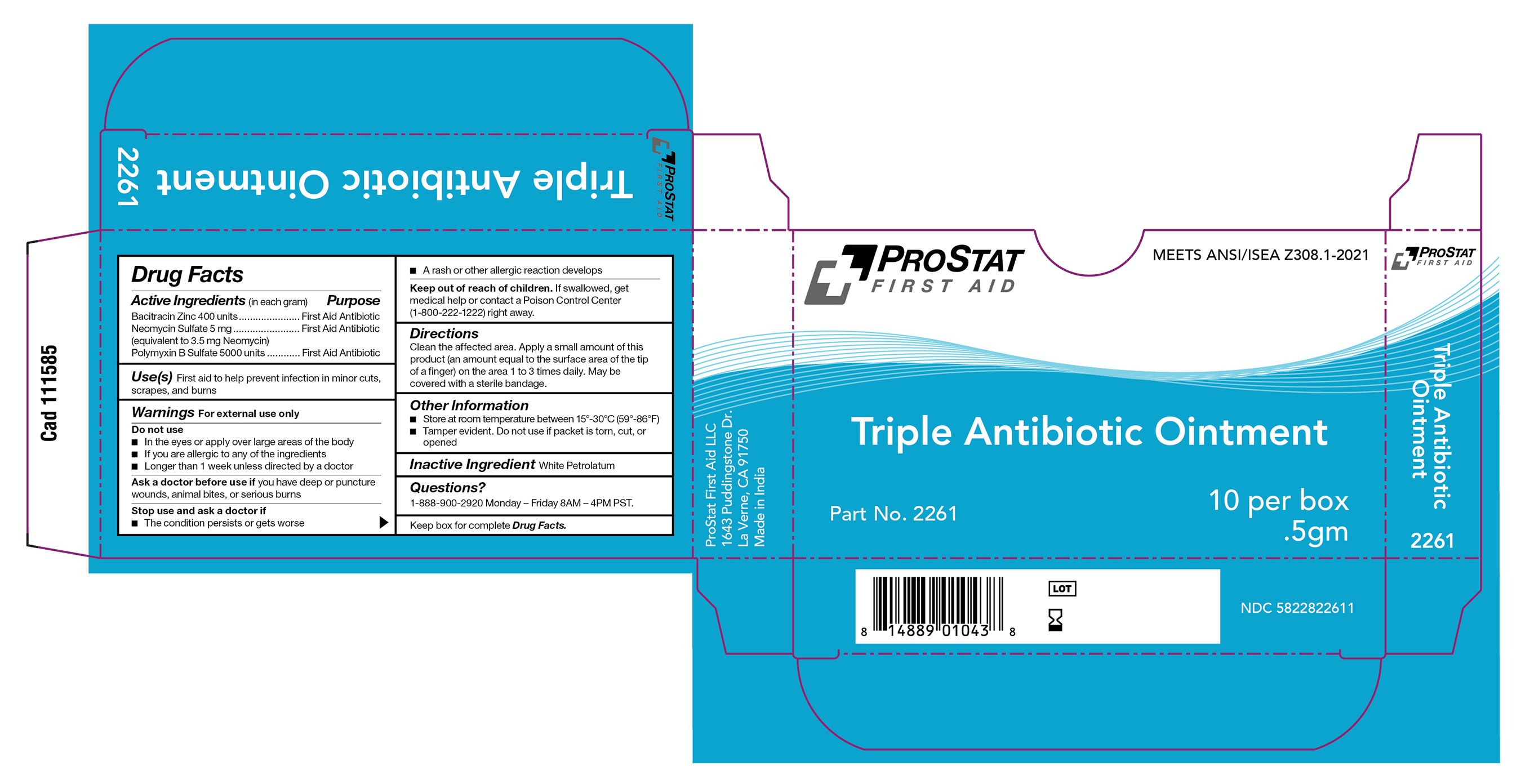
Label: PS-2261 TRIPLE ANTIBIOTIC, 0.5G ointment
- NDC Code(s): 58228-2261-1
- Packager: ProStat First Aid LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 14, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Use(s)
- Warnings
- Directions
- Other Information
- Inactive Ingredient
- Questions
- Label
-
INGREDIENTS AND APPEARANCE
PS-2261 TRIPLE ANTIBIOTIC, 0.5G
ps-2261 triple antibiotic, 0.5g ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58228-2261 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 5 mg in 1 g Inactive Ingredients Ingredient Name Strength WHITE PETROLATUM (UNII: B6E5W8RQJ4) Product Characteristics Color Score Shape FREEFORM Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58228-2261-1 1000 in 1 CARTON 07/31/2023 1 10 in 1 BOX 1 0.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 07/31/2023 Labeler - ProStat First Aid LLC (061263699) Registrant - Dynarex Corporation (008124539)

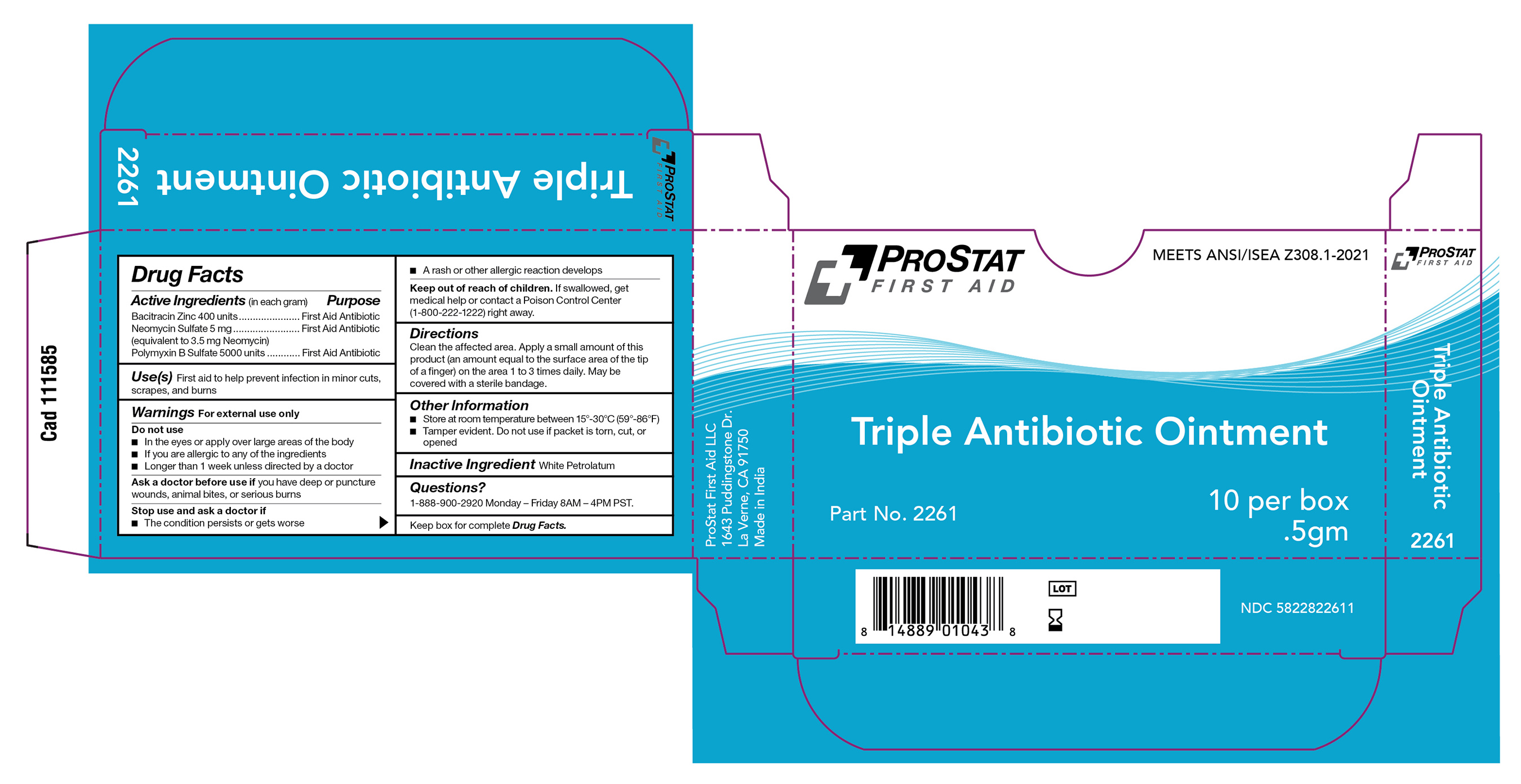
 PS-2261 Triple Antibiotic, 0.5g
PS-2261 Triple Antibiotic, 0.5g