Label: AKLIEF- trifarotene cream
- NDC Code(s): 0299-5935-02, 0299-5935-30, 0299-5935-45, 0299-5935-75
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AKLIEF - ®Cream safely and effectively. See full prescribing information for AKLIEF Cream. AKLIEF(trifarotene) cream, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAKLIEF Cream is a retinoid indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
-
2 DOSAGE AND ADMINISTRATIONApply a thin layer of AKLIEF Cream to the affected areas once daily, in the evening, on clean and dry skin. One pump actuation should be enough to cover the face (i.e., forehead, cheeks ...
-
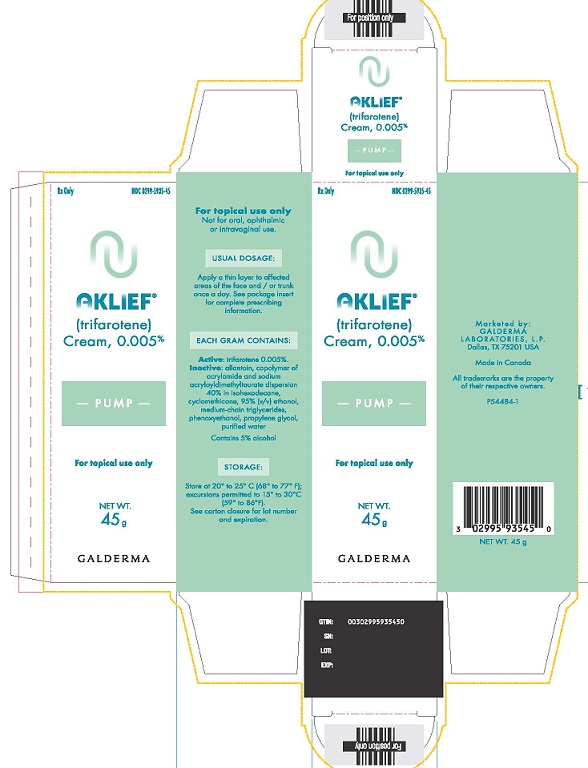
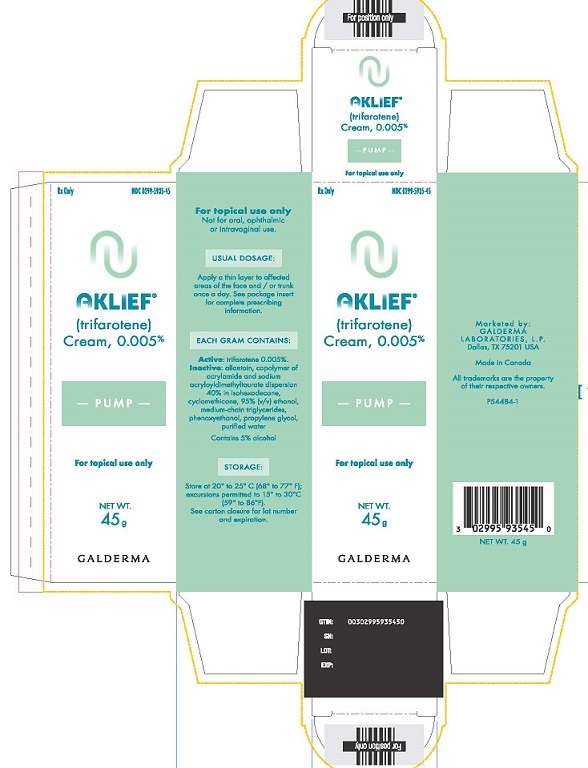
3 DOSAGE FORMS AND STRENGTHSCream: 0.005%. Each gram of AKLIEF Cream contains 50 mcg of trifarotene in a white cream.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Skin Irritation - Patients using AKLIEF Cream may experience erythema, scaling, dryness, and stinging/burning. Maximum severity of these reactions typically occurred within the first 4 weeks ...
-
6 ADVERSE REACTIONS6.1 Clinical trials experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSTopical application of AKLIEF Cream is not expected to affect the circulating concentrations of oral hormonal contraceptives containing ethinyl estradiol and levonorgestrel.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from clinical trials with AKLIEF Cream use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or ...
-
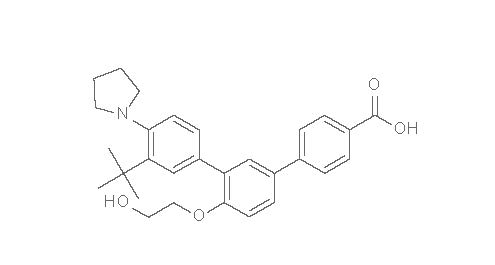
11 DESCRIPTIONAKLIEF Cream for topical administration contains 0.005% (50 mcg/g) trifarotene. Trifarotene is a terphenyl acid derivative and is a retinoid. The chemical name of trifarotene is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Trifarotene is an agonist of retinoic acid receptors (RAR), with particular activity at the gamma subtype of RAR. Stimulation of RAR results in modulation of target ...
-
13. NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Trifarotene was not carcinogenic when topically applied to mice daily for up to 24 months in the vehicle of the product (AKLIEF Cream ...
-
14 CLINICAL STUDIESAKLIEF Cream applied once daily in the evening was evaluated in the treatment of moderate facial and truncal acne vulgaris in two randomized, multicenter, parallel group, double-blind ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAKLIEF Cream, 0.005% is provided as a white cream supplied in the following packaging configurations with corresponding NDC numbers: 45-gram pump NDC ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information). Advise the patient to: Cleanse the area to be treated; pat dry. Apply AKLIEF Cream as a thin layer once daily in the evening to the ...
- PATIENT PACKAGE INSERT
-
PACKAGE LABEL 45g CARTONRX only - NDC 0299-5935-45 - AKLIEF - (trifarotene) Cream, 0.005% PUMP - For topical use only - NET WT. 45 g - GALDERMA - For topical use ...
-
INGREDIENTS AND APPEARANCEProduct Information