Label: OSMOPREP- sodium phosphate, monobasic, monohydrate, sodium phosphate, dibasic anhydrous tablet
-
Contains inactivated NDC Code(s)
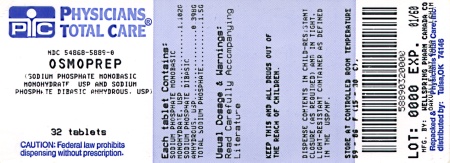
NDC Code(s): 54868-5889-0, 54868-5889-1 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 65649-701
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 4, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNINGS
There have been rare, but serious reports of acute phosphate nephropathy in patients who received oral sodium phosphate products for colon cleansing prior to colonoscopy. Some cases have resulted in permanent impairment of renal function and some patients required long-term dialysis. While some cases have occurred in patients without identifiable risk factors, patients at increased risk of acute phosphate nephropathy may include those with increased age, hypovolemia, increased bowel transit time (such as bowel obstruction), active colitis, or baseline kidney disease, and those using medicines that affect renal perfusion or function (such as diuretics, angiotensin converting enzyme [ACE] inhibitors, angiotensin receptor blockers [ARBs], and possibly nonsteriodal anti-inflammatory drugs [NSAIDs]).
Close
See WARNINGS.
It is important to use the dose and dosing regimen as recommended (pm/am split dose).
See DOSAGE and ADMINISTRATION. -
DESCRIPTIONOsmoPrep® (sodium phosphate monobasic - monohydrate, USP, and sodium phosphate dibasic anhydrous, USP) is a purgative - used to clean the colon prior to colonoscopy. OsmoPrep is manufactured with a ...
-
CLINICAL PHARMACOLOGYOsmoPrep Tablets, a dosing regimen containing 48 grams of sodium - phosphate (32 tablets), induces diarrhea, which effectively cleanses the entire - colon. Each administration has a purgative effect ...
-
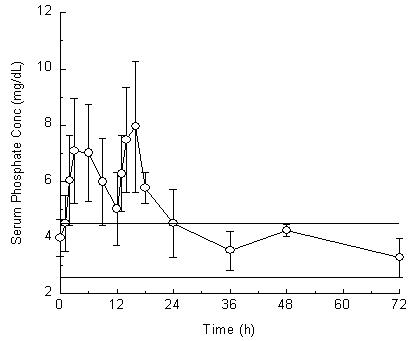
CLINICAL STUDIESThe colon cleansing efficacy and safety of OsmoPrep was evaluated - in 2 randomized, investigator-blinded, actively controlled, multicenter, U.S. trials in patients scheduled to have an elective ...
-
INDICATIONS AND USAGEOsmoPrep Tablets are indicated for cleansing of the colon as a - preparation for colonoscopy in adults 18 years of age or older.
-
CONTRAINDICATIONSOsmoPrep Tablets are contraindicated in patients with - biopsy-proven acute phosphate nephropathy. OsmoPrep Tablets are contraindicated in patients with a known allergy or - hypersensitivity to ...
-
WARNINGSAdministration of sodium phosphate products prior to colonoscopy - for colon cleansing has resulted in fatalities due to significant fluid shifts, severe electrolyte abnormalities, and cardiac ...
-
PRECAUTIONSGeneralPatients should be instructed to drink 8 ounces of clear liquids - with each 4-tablet dose of OsmoPrep Tablets. Patients should take a total of 2 - quarts of clear liquids with OsmoPrep ...
-
ADVERSE REACTIONSAbdominal bloating, abdominal pain, nausea, and vomiting were the - most common adverse events reported with the use of OsmoPrep Tablets. Dizziness - and headache were reported less frequently ...
-
DRUG ABUSE AND DEPENDENCELaxatives and purgatives (including OsmoPrep) have the potential for abuse by - bulimia nervosa patients who frequently have binge eating and vomiting.
-
OVERDOSAGEThere have been no reported cases of overdosage with OsmoPrep Tablets. Purposeful or accidental ingestion of more than the recommended dosage of - OsmoPrep Tablets might be expected to lead to ...
-
DOSAGE AND ADMINISTRATIONThe recommended dose of OsmoPrep Tablets for colon cleansing for - adult patients is 32 tablets (48 grams of sodium phosphate) taken orally with a - total of 2 quarts of clear liquids in the ...
-
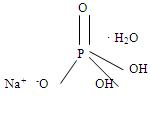
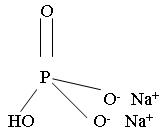
HOW SUPPLIEDOsmoPrep Tablets are supplied in child-resistant bottles - containing 32 tablets. Each tablet contains 1.102 g sodium phosphate monobasic - monohydrate, USP and 0.398 g sodium phosphate dibasic ...
-
MEDICATION GUIDEMedication Guide - OsmoPrep® (AhZ-MŌ-prěp) (sodium phosphate monobasic monohydrate, USP - and sodium phosphate dibasic anhydrous, USP) Tablets - Read the Medication Guide that comes with OsmoPrep ...
-
PRINCIPAL DISPLAY PANELOsmoPrep® (sodium phosphate monobasic monohydrate, USP - and sodium phosphate dibasic anhydrous, USP) Tablets
-
INGREDIENTS AND APPEARANCEProduct Information