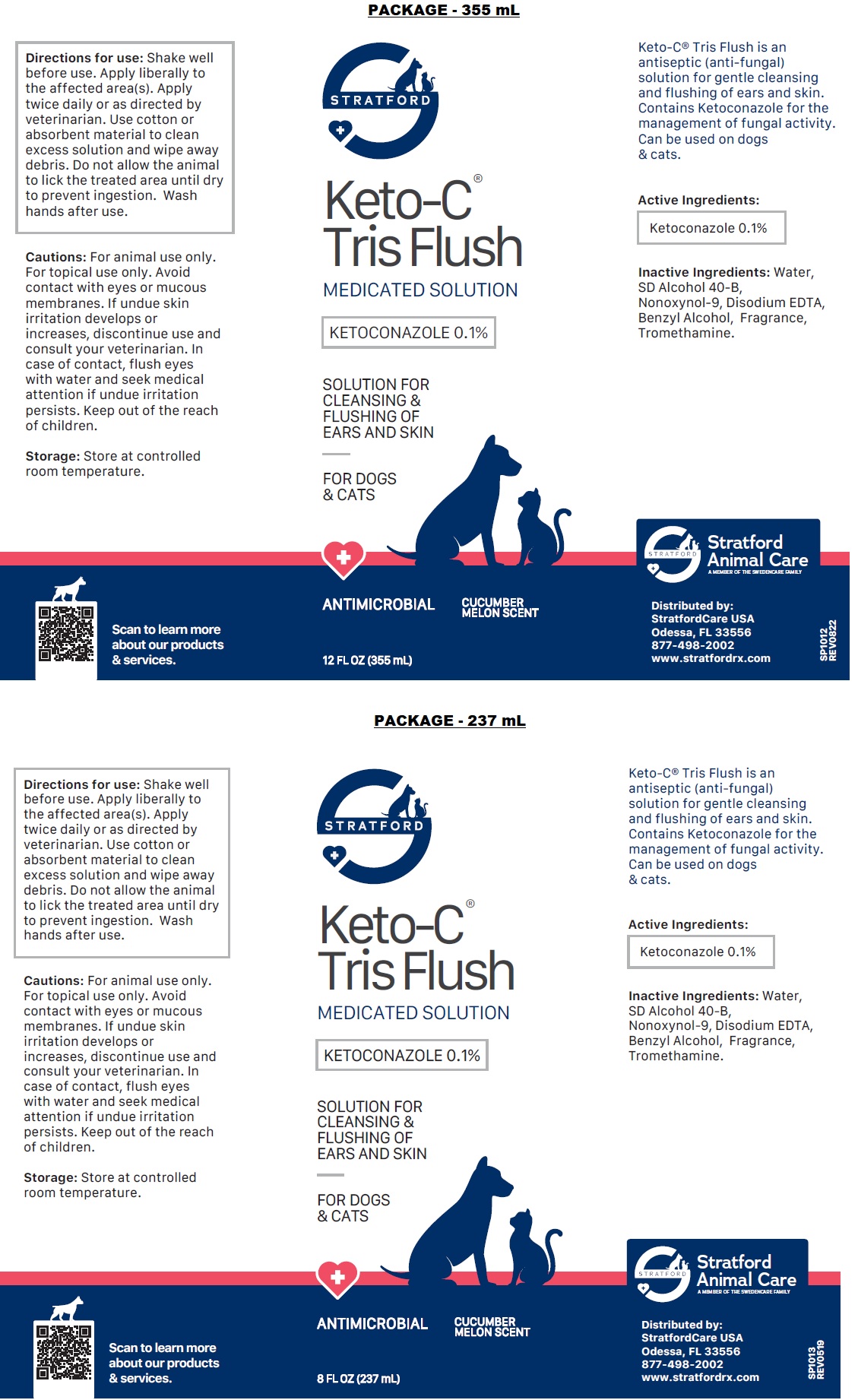
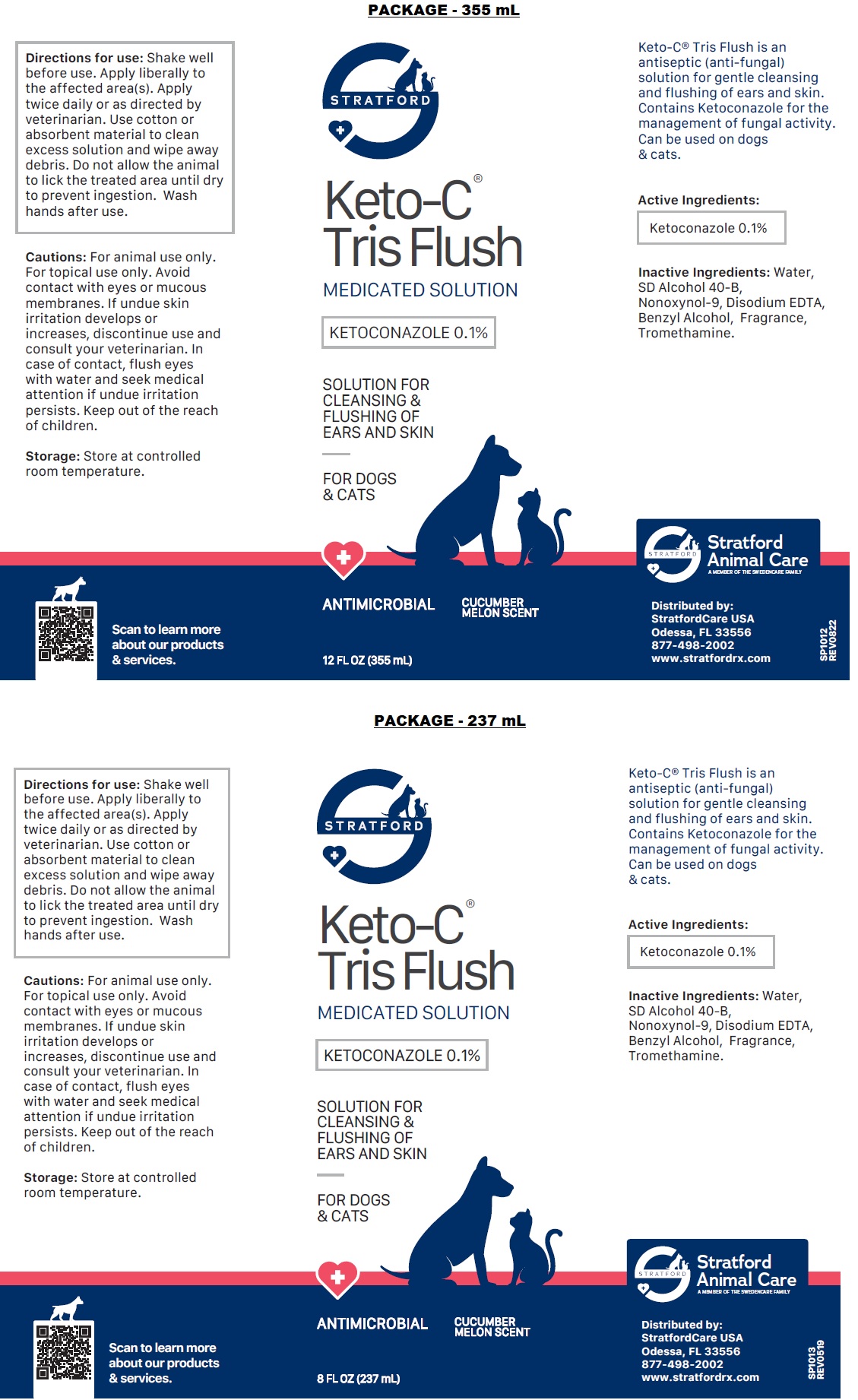
Label: KETO-C-TRIS FLUSH- ketoconazole solution
- NDC Code(s): 86069-111-04, 86069-111-08, 86069-111-12
- Packager: Stratford Care Usa, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 10, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Directions for use: Shake well before use. Apply liberally to the affected area(s). Apply twice daily or as directed by veterinarian. Use cotton or absorbent material to clean excess solution and wipe away debris. Do not allow the animal to lick the treated area until dry to prevent ingestion. Wash hands after use.
-
PRECAUTIONS
Cautions: For animal use only. For topical use only. Avoid contact with eyes or mucous membranes. If undue skin irritation develops or increases, discontinue use and consult your veterinarian. In case of contact, flush eyes with water and seek medical attention if undue irritation persists. Keep out of the reach of children.
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
KETO-C-TRIS FLUSH
ketoconazole solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86069-111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOCONAZOLE (UNII: R9400W927I) (KETOCONAZOLE - UNII:R9400W927I) KETOCONAZOLE 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) NONOXYNOL-9 (UNII: 48Q180SH9T) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) BENZYL ALCOHOL (UNII: LKG8494WBH) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86069-111-12 355 mL in 1 CONTAINER 2 NDC:86069-111-04 118 mL in 1 BOTTLE, PLASTIC 3 NDC:86069-111-08 237 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2015 Labeler - Stratford Care Usa, Inc. (036650469)