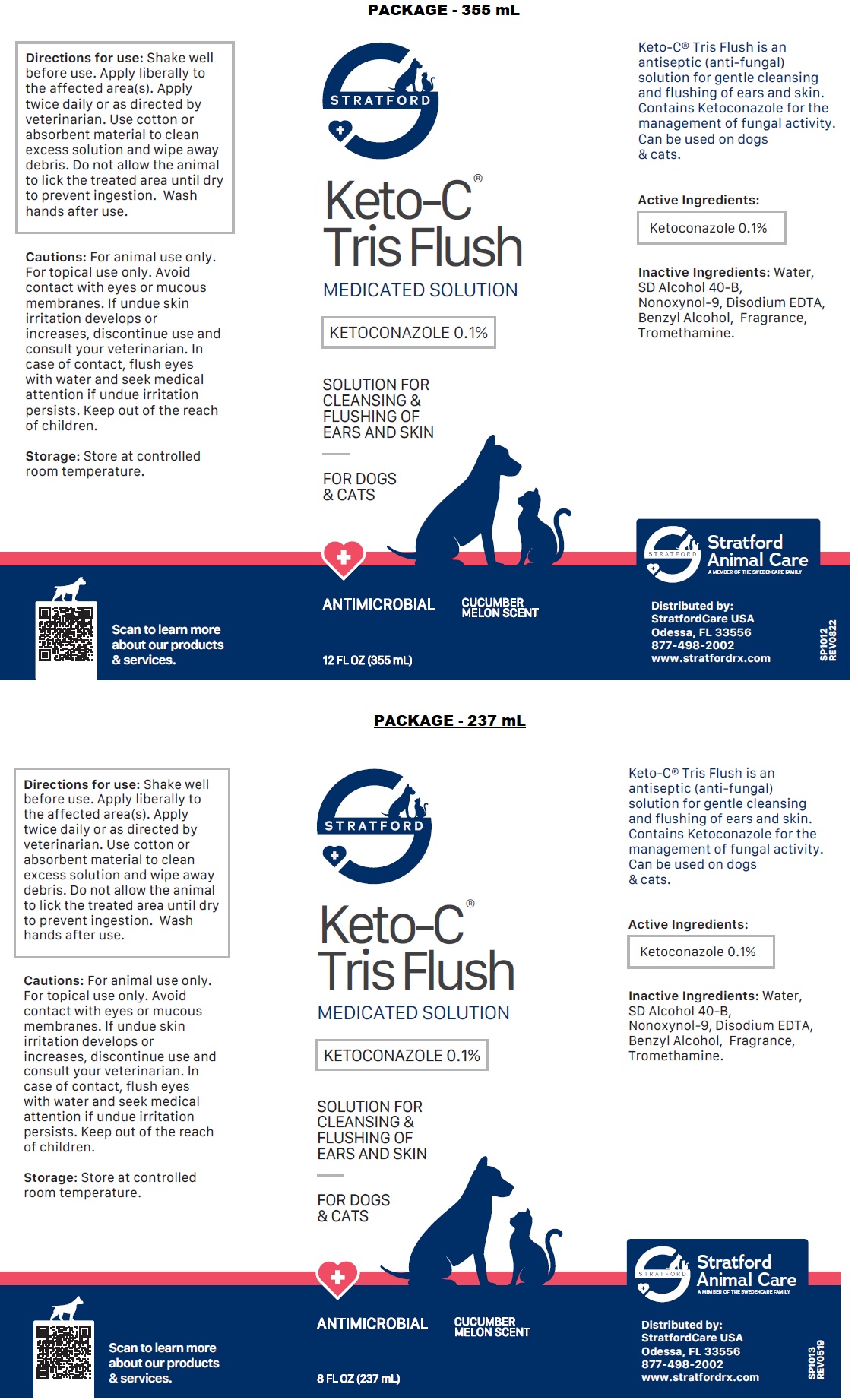
Keto-C® Tris Flush is an antiseptic (anti-fungal) solution for gentle cleansing and flushing of ears and skin. Contains Ketoconazole for the management of fungal activity. Can be used on dogs & cats.
Inactive Ingredients: Water, SD Alcohol 40-B, Nonoxynol-9, Disodium EDTA, Benzyl Alcohol, Fragrance, Tromethamine.
Directions for use: Shake well before use. Apply liberally to the affected area(s). Apply twice daily or as directed by veterinarian. Use cotton or absorbent material to clean excess solution and wipe away debris. Do not allow the animal to lick the treated area until dry to prevent ingestion. Wash hands after use.
Cautions: For animal use only. For topical use only. Avoid contact with eyes or mucous membranes. If undue skin irritation develops or increases, discontinue use and consult your veterinarian. In case of contact, flush eyes with water and seek medical attention if undue irritation persists. Keep out of the reach of children.