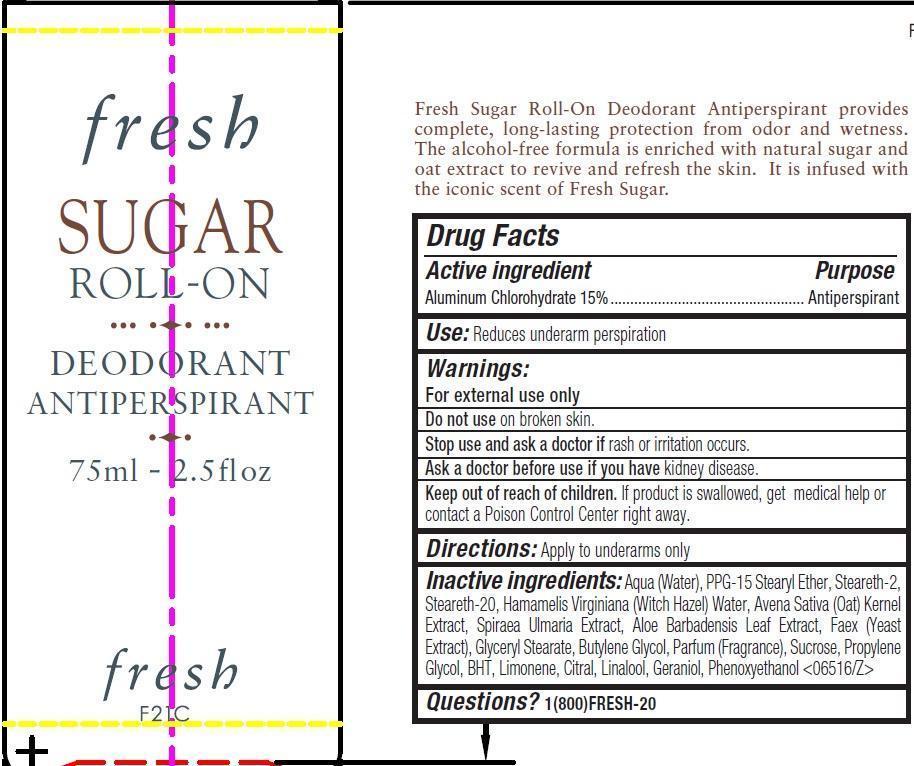
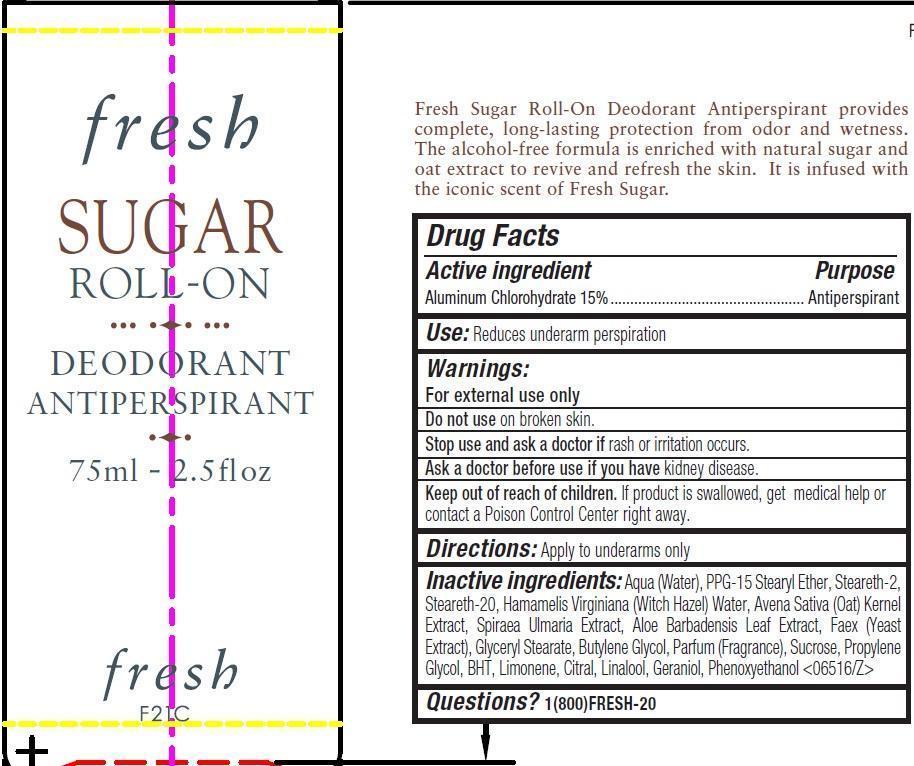
Label: FRESH SUGAR ROLL-ON DEODORANT- aluminum chlorohydrate emulsion
- NDC Code(s): 42406-014-00
- Packager: Fresh Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use:
- Warnings:
- Directions:
-
Inactive ingredients:
Aqua (Water), PPG-15 Stearyl Ether, Steareth-2, Steareth-20, Hamamelis Virginiana (Witch Hazel) Water, Avena Sativa (Oat) Kernel Extract, Spiraea Ulmaria Extract, Aloe Barbadensis Leaf Extract, Faex (Yeast Extract), Glyceryl Stearate, Butylene Glycol, Parfum (Fragrance), Sucrose, Propylene Glycol, BHT, Limonene, Citral, Linalool, Geraniol, Phenoxyethanol <06516/Z>
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
FRESH SUGAR ROLL-ON DEODORANT
aluminum chlorohydrate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42406-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength PPG-15 STEARYL ETHER (UNII: 1II18XLS1L) STEARETH-2 (UNII: V56DFE46J5) STEARETH-20 (UNII: L0Q8IK9E08) OAT (UNII: Z6J799EAJK) ALOE VERA LEAF (UNII: ZY81Z83H0X) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SUCROSE (UNII: C151H8M554) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CITRAL (UNII: T7EU0O9VPP) LINALOOL, (+/-)- (UNII: D81QY6I88E) GERANIOL (UNII: L837108USY) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42406-014-00 1 in 1 BOX 09/18/2015 10/01/2024 1 75 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 09/18/2015 10/01/2024 Labeler - Fresh Inc. (021403729)