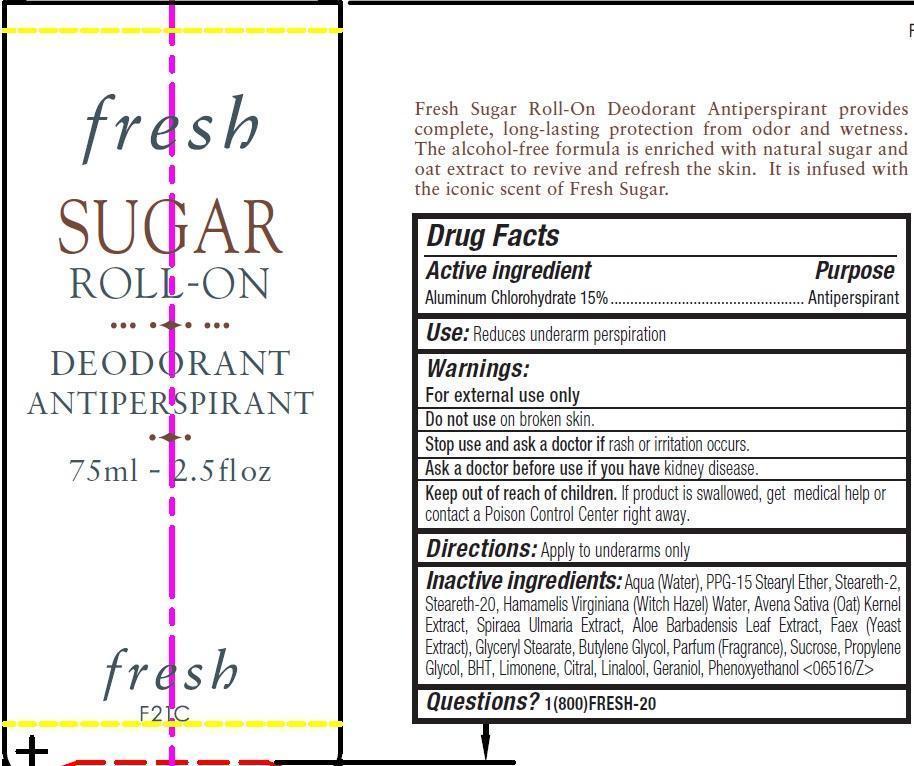
Active ingredient
Aluminum Chlorohydrate 15%
Use:
Reduces underarm perspiration
Warnings:
For external use only
Do not use
on broken skin.
Stop use and ask a doctor if
rash or irritation occurs.
Ask a doctor before use if you have
kidney disease.
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions:
Apply to underarms only
Inactive ingredients:
Aqua (Water), PPG-15 Stearyl Ether, Steareth-2, Steareth-20, Hamamelis Virginiana (Witch Hazel) Water, Avena Sativa (Oat) Kernel Extract, Spiraea Ulmaria Extract, Aloe Barbadensis Leaf Extract, Faex (Yeast Extract), Glyceryl Stearate, Butylene Glycol, Parfum (Fragrance), Sucrose, Propylene Glycol, BHT, Limonene, Citral, Linalool, Geraniol, Phenoxyethanol <06516/Z>
Package Labeling:
Fresh Inc.
