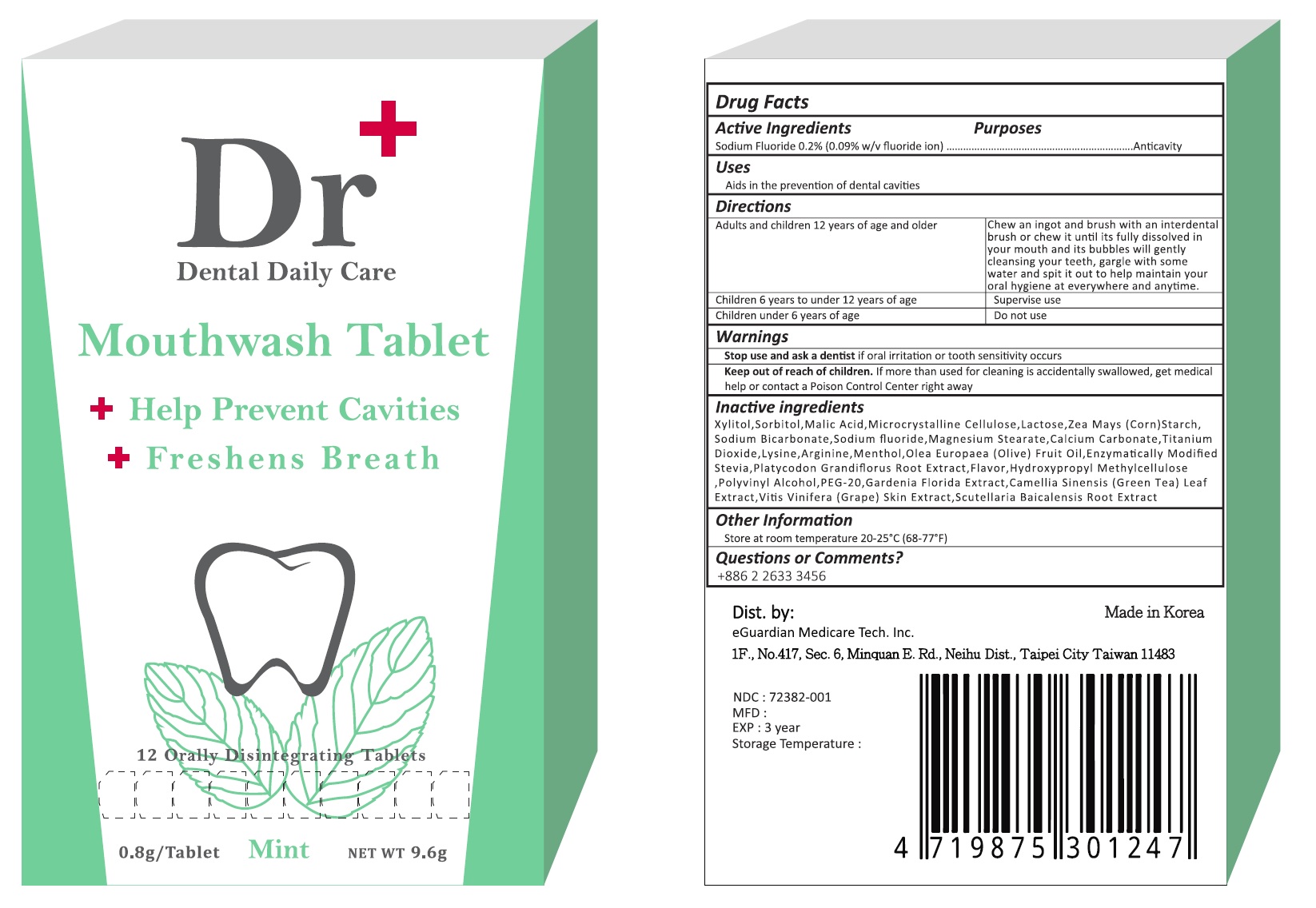
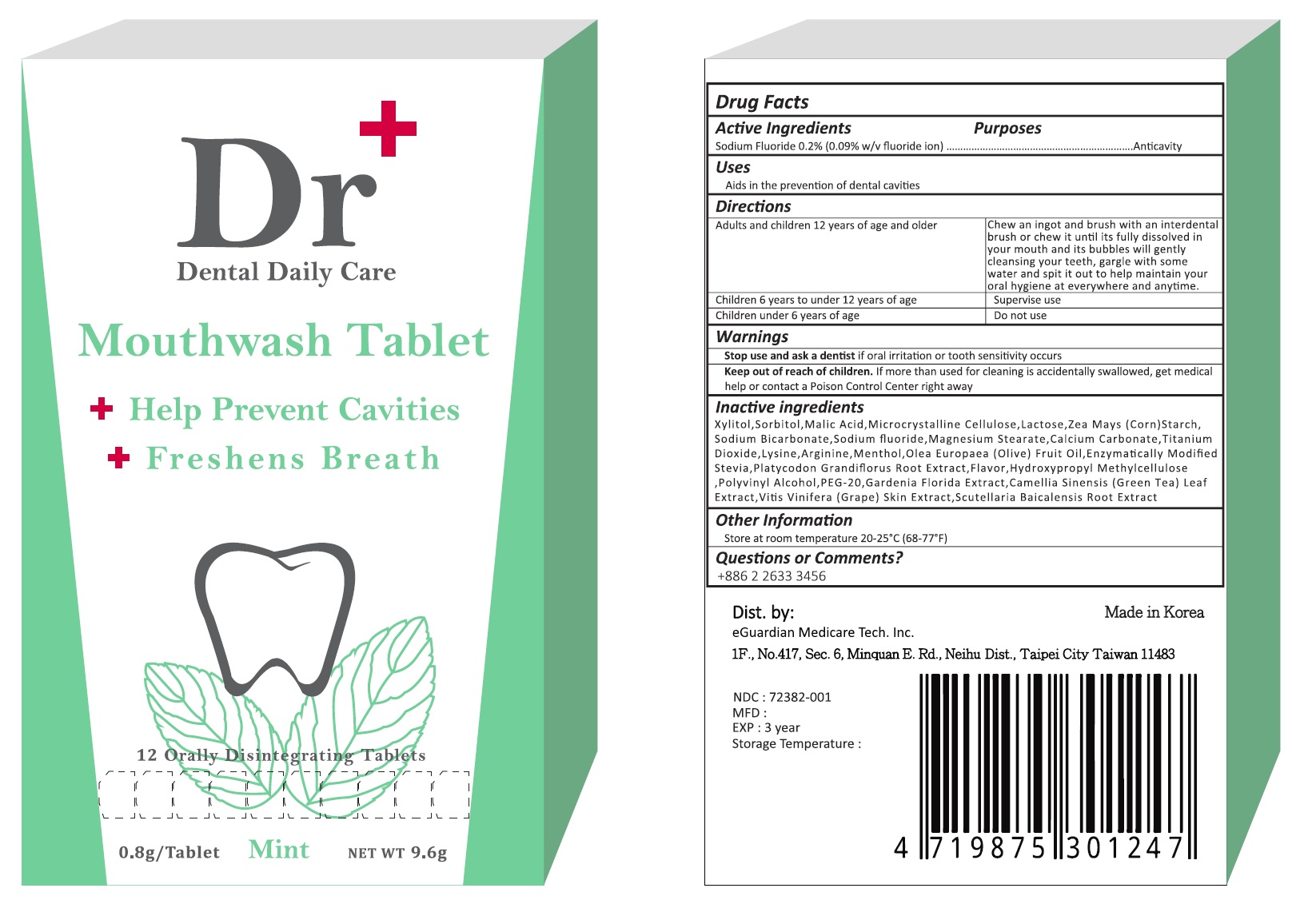
Label: DR PLUS DENTAL DAILY CARE- sodium fluoride tablet, orally disintegrating
-
Contains inactivated NDC Code(s)
NDC Code(s): 72382-001-01, 72382-001-02, 72382-001-03, 72382-001-04, view more72382-001-05, 72382-001-06 - Packager: EGUARDIAN MEDICARE TECH INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 14, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
adults and children 12 years and older - Chew an ingot and brush with an interdental brush or chew it until its fully dissolved in your mouth and its bubbles will gently cleansing your teeth, gargle with some water and spit it out to help maintain your oral hygiene at everywhere and anytime.
children 6 years to under 12 years of age - Supervise use.
children under 6 years - Do not use
-
INACTIVE INGREDIENT
Inactive ingredients
Xylitol, Sorbitol, Malic Acid, Microcrystalline Cellulose, Lactose, Zea Mays (Corn) Starch, Sodium Bicarbonate, Magnesium Steartae, Calcium Carbonate, Titanium Dioxide, Lysine, Arginine, Menthol, Olea Europaea (Olive) Fruit Oil, Enzymatically Modified Stevia, Platycodon Grandiflorus Root Extract, Flavor, Hydroxypropyle Methylcellulose, Polyvinyl Alcohol, PEG-20, Gardenia Floride Extract, Camellia Sinensis (Green Tea) Leaf Extract, Vitis Vinifera (Grape) Skin Extract, Scutellaria Baicalensis Root Extract
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR PLUS DENTAL DAILY CARE
sodium fluoride tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72382-001 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 2 mg in 1 g Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) SORBITOL (UNII: 506T60A25R) MALIC ACID (UNII: 817L1N4CKP) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) STARCH, CORN (UNII: O8232NY3SJ) SODIUM BICARBONATE (UNII: 8MDF5V39QO) MAGNESIUM STEARATE (UNII: 70097M6I30) CALCIUM CARBONATE (UNII: H0G9379FGK) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) LYSINE (UNII: K3Z4F929H6) ARGININE (UNII: 94ZLA3W45F) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) OLIVE OIL (UNII: 6UYK2W1W1E) PLATYCODON GRANDIFLORUS ROOT (UNII: 2DF0NS0O2Z) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) GARDENIA JASMINOIDES WHOLE (UNII: 0PK353KHF0) GREEN TEA LEAF (UNII: W2ZU1RY8B0) VITIS VINIFERA ANTHOCYANINS (UNII: F02KPB2508) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) Product Characteristics Color green (Light Green) Score no score Shape RECTANGLE Size 11mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72382-001-03 1 in 1 CARTON 08/14/2018 1 NDC:72382-001-02 12 in 1 BLISTER PACK 1 NDC:72382-001-01 0.8 g in 1 CAPSULE; Type 0: Not a Combination Product 2 NDC:72382-001-06 1 in 1 CARTON 08/14/2018 2 NDC:72382-001-05 30 in 1 BLISTER PACK 2 NDC:72382-001-04 0.8 g in 1 CAPSULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 08/14/2018 Labeler - EGUARDIAN MEDICARE TECH INC. (656394704) Registrant - EGUARDIAN MEDICARE TECH INC. (656394704) Establishment Name Address ID/FEI Business Operations JUHWANBIO.CELL CO.,LTD. 690163444 manufacture(72382-001)