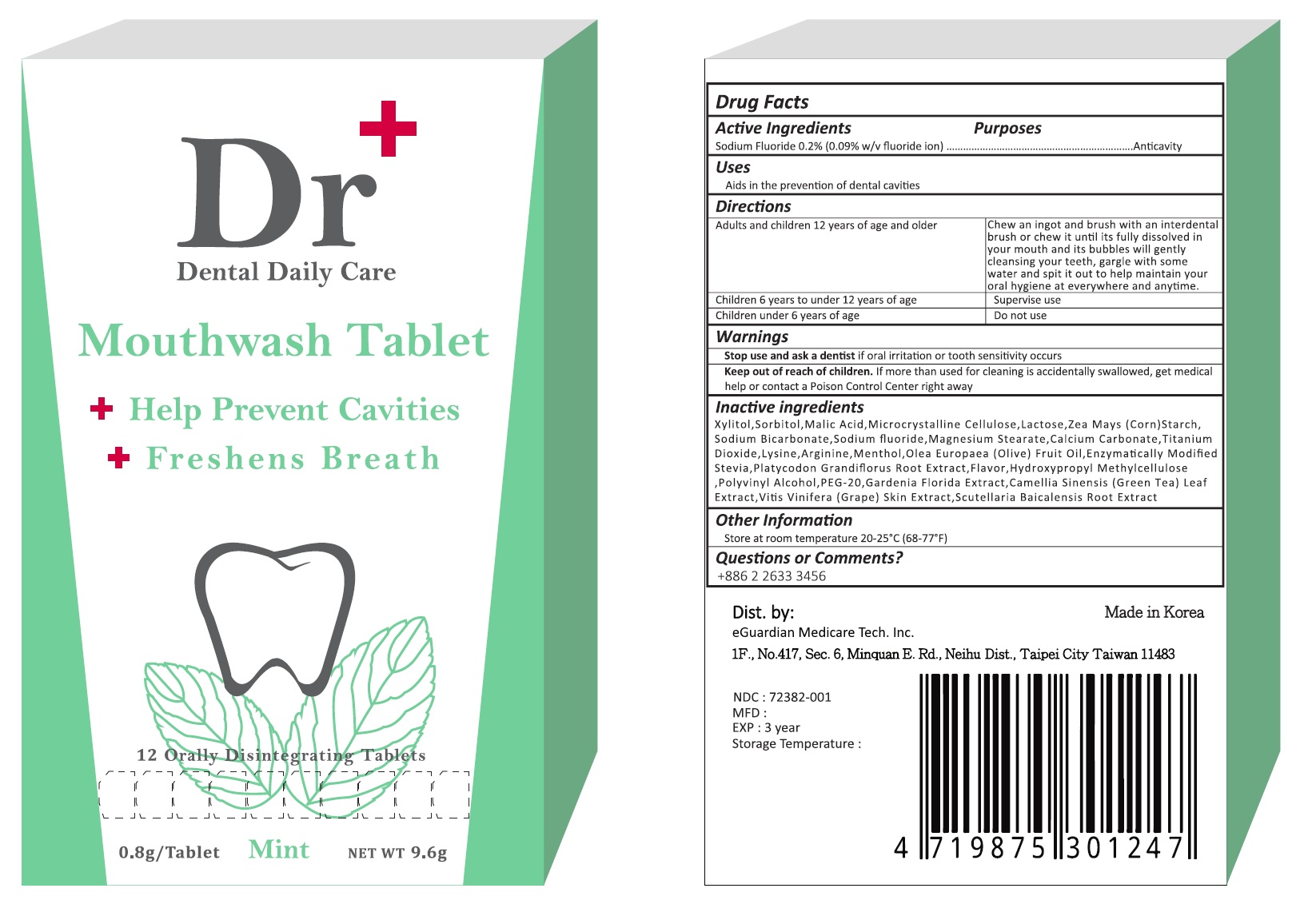
Keep out of reach of children. If more than used for cleaning is accidentally swallowed, get medical help or contact a Poison Control Center right away.
adults and children 12 years and older - Chew an ingot and brush with an interdental brush or chew it until its fully dissolved in your mouth and its bubbles will gently cleansing your teeth, gargle with some water and spit it out to help maintain your oral hygiene at everywhere and anytime.
children 6 years to under 12 years of age - Supervise use.
children under 6 years - Do not use
Inactive ingredients
Xylitol, Sorbitol, Malic Acid, Microcrystalline Cellulose, Lactose, Zea Mays (Corn) Starch, Sodium Bicarbonate, Magnesium Steartae, Calcium Carbonate, Titanium Dioxide, Lysine, Arginine, Menthol, Olea Europaea (Olive) Fruit Oil, Enzymatically Modified Stevia, Platycodon Grandiflorus Root Extract, Flavor, Hydroxypropyle Methylcellulose, Polyvinyl Alcohol, PEG-20, Gardenia Floride Extract, Camellia Sinensis (Green Tea) Leaf Extract, Vitis Vinifera (Grape) Skin Extract, Scutellaria Baicalensis Root Extract