Label: ANTICAVITY FLUORIDE RINSE- sodium fluoride mouthwash
- NDC Code(s): 71141-159-32
- Packager: Lidl US, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Use
- Warnings
-
Directions
Adults and children 6 years of age and older:
- use twice daily after brushing your teeth with a toothpaste
- vigorously swish 10 ml (2 teaspoonfuls) of rinse between your teeth for 1 minute and then spit it out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- instruct children under 12 years of age in good rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
children under 6 years of age: consult a dentist or doctor
- Other information
- Inactive ingredients
- Questions?
- Disclaimer
- Adverse Reactions
-
Principal display panel
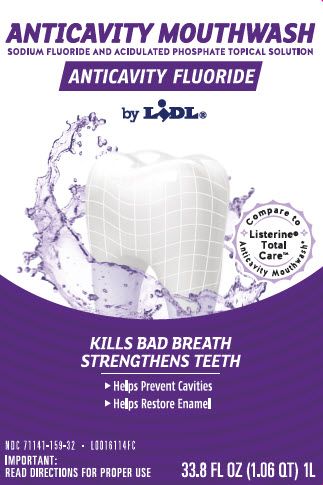
ANTICAVITY MOUTHWASH
SODIUM FLUORIDE AND ACIDULATED PHOSPHATE TOPICAL SOLUTION
ANTICAVITY FLUORIDE
by LIDL
COMPARE TO LISTERINE TOTAL CARE ANTICAVITY MOUTHWASH
Kills Bad Breath and Strengthens Teeth to Help Prevent Cavities
- Helps restore enemal
- Helps prevent cavities
IMPORTANT: read directions for proper use
33.8 FL OZ (1.06 QT) 1L
-
principal display panel
dentalux
by LIDL MUTLI-CARE
SODIUM FLUORIDE AND ACIDULATED PHOSPHATE TOPICAL SOLUTION
ANTIICAVITY FLUORIDE MOUTHWASH
Compare to Listerine Total Care Anticavity Mouthwash*
- Kills bad breath
- Strengthen teeth
- Helps resotre enamel
- Helps prevent cavities
Sealed with printed neckband for your protection
Helps prevent cavities, restores enamel, & kills bad breath germs
NDC-71141-159-32
33.8 FL OZ (1.06 QT) 1L

-
INGREDIENTS AND APPEARANCE
ANTICAVITY FLUORIDE RINSE
sodium fluoride mouthwashProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71141-159 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) SORBITOL (UNII: 506T60A25R) POLOXAMER 407 (UNII: TUF2IVW3M2) EUCALYPTOL (UNII: RV6J6604TK) METHYL SALICYLATE (UNII: LAV5U5022Y) MENTHOL (UNII: L7T10EIP3A) PHOSPHORIC ACID (UNII: E4GA8884NN) SACCHARIN SODIUM (UNII: SB8ZUX40TY) THYMOL (UNII: 3J50XA376E) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SUCRALOSE (UNII: 96K6UQ3ZD4) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71141-159-32 1000 mL in 1 PACKAGE; Type 0: Not a Combination Product 02/09/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 02/09/2017 Labeler - Lidl US, LLC (079389709) Registrant - Vi-Jon, LLC (790752542) Establishment Name Address ID/FEI Business Operations Vi-Jon, LLC 790752542 manufacture(71141-159) Establishment Name Address ID/FEI Business Operations Vi-Jon, LLC 088520668 manufacture(71141-159)