Directions
Adults and children 6 years of age and older:
- use twice daily after brushing your teeth with a toothpaste
- vigorously swish 10 ml (2 teaspoonfuls) of rinse between your teeth for 1 minute and then spit it out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- instruct children under 12 years of age in good rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
children under 6 years of age: consult a dentist or doctor
Other information
- store at controlled room temperature 20⁰ - 25⁰ C (68⁰ - 77⁰ F)
- cold weather may temporarily cloud this product
Inactive ingredients
water, alcohol (21.6 %v/v), sorbitol, poloxamer 407, eucalyptol, flavor, methyl salicylate, menthol, phosphoric acid, sodium saccharin, thymol, disodium phosphate, sucralose, red 40, blue 1
Disclaimer
This product is not manufactured or distributed by Johnson & Johnson Corporation, owner of the registered trademark Listerine
Adverse Reactions
DISTRIBUTED BY
Lidl US, LLC
Arlington, VA 22202
1-844-344-5071
www.Lidl.com
Lidl Love it! Guarantee
- money refunded
- item replaced
save water.lidl
how2recycle.info
Discard Seal, Empty & Replace Cap
Plastic Bottle
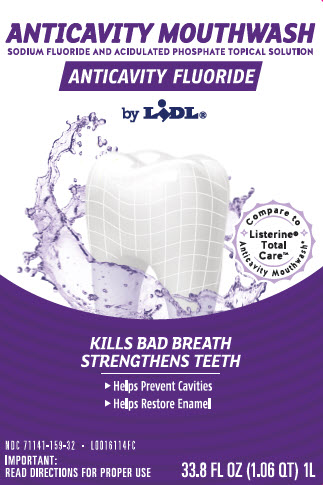
Principal display panel
ANTICAVITY MOUTHWASH
SODIUM FLUORIDE AND ACIDULATED PHOSPHATE TOPICAL SOLUTION
ANTICAVITY FLUORIDE
by LIDL
COMPARE TO LISTERINE TOTAL CARE ANTICAVITY MOUTHWASH
Kills Bad Breath and Strengthens Teeth to Help Prevent Cavities
- Helps restore enemal
- Helps prevent cavities
IMPORTANT: read directions for proper use
33.8 FL OZ (1.06 QT) 1L
principal display panel
dentalux
by LIDL MUTLI-CARE
SODIUM FLUORIDE AND ACIDULATED PHOSPHATE TOPICAL SOLUTION
ANTIICAVITY FLUORIDE MOUTHWASH
Compare to Listerine Total Care Anticavity Mouthwash*
- Kills bad breath
- Strengthen teeth
- Helps resotre enamel
- Helps prevent cavities
Sealed with printed neckband for your protection
Helps prevent cavities, restores enamel, & kills bad breath germs
NDC-71141-159-32
33.8 FL OZ (1.06 QT) 1L
