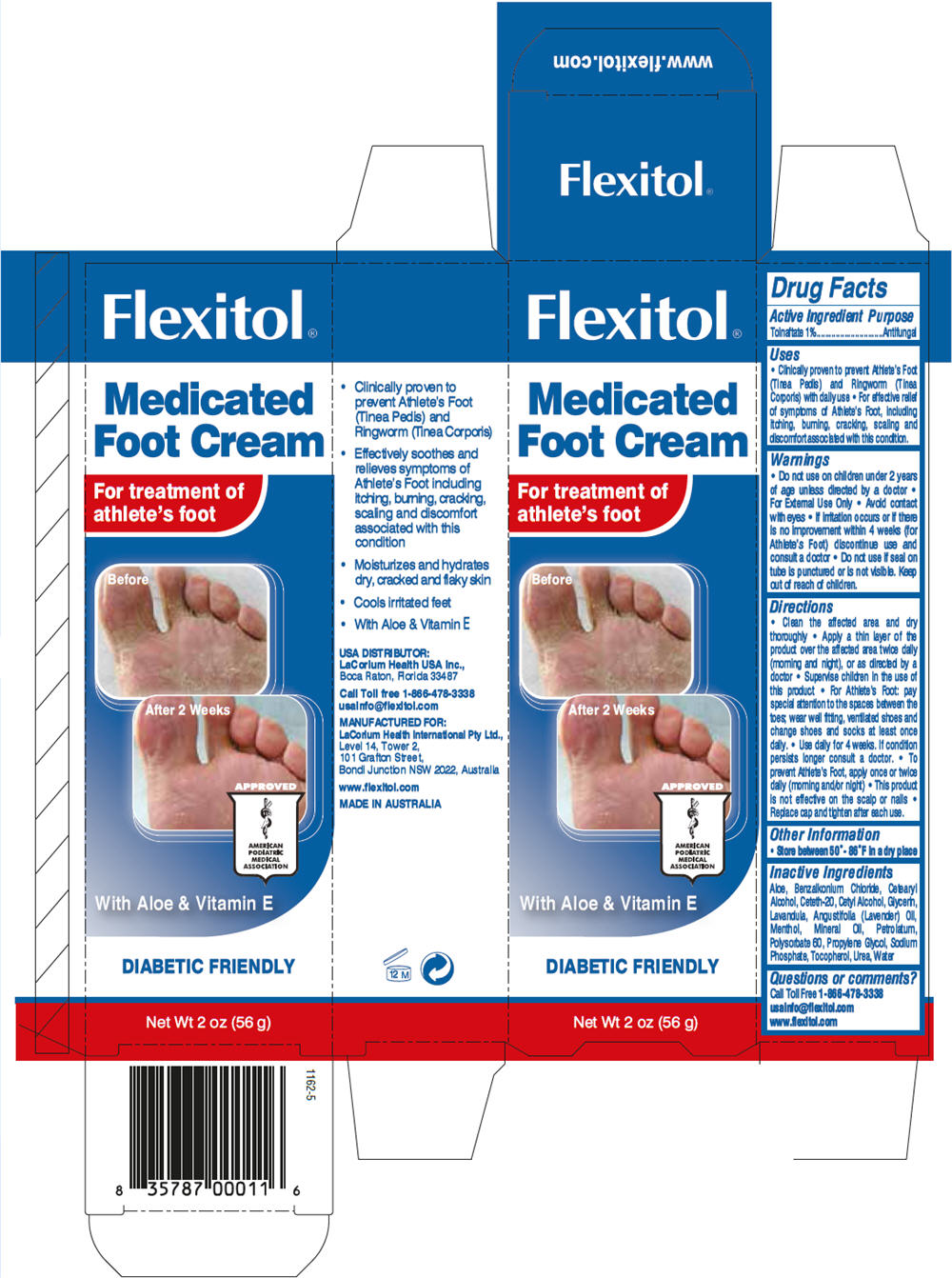
Label: FLEXITOL MEDICATED FOOT- tolnaftate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 43251-3340-1 - Packager: LaCorium Health International Pty Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 12, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
-
Directions
- Clean the affected area and dry thoroughly
- Apply a thin layer of the product over the affected area twice daily (morning and night), or as directed by a doctor
- Supervise children in the use of this product
- For Athlete's Foot: pay special attention to the spaces between the toes; wear well fitting, ventilated shoes and change shoes and socks at least once daily.
- Use daily for 4 weeks. If condition persists longer consult a doctor.
- To prevent Athlete's Foot, apply once or twice daily (morning and/or night)
- This product is not effective on the scalp or nails
- Replace cap and tighten after each use.
- Other Information
- Inactive Ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 56 g Tube Carton
-
INGREDIENTS AND APPEARANCE
FLEXITOL MEDICATED FOOT
tolnaftate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43251-3340 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Tolnaftate (UNII: 06KB629TKV) (Tolnaftate - UNII:06KB629TKV) Tolnaftate 1 g in 100 g Inactive Ingredients Ingredient Name Strength Urea (UNII: 8W8T17847W) Cetostearyl Alcohol (UNII: 2DMT128M1S) Mineral Oil (UNII: T5L8T28FGP) Glycerin (UNII: PDC6A3C0OX) Propylene glycol (UNII: 6DC9Q167V3) Petrolatum (UNII: 4T6H12BN9U) Polysorbate 60 (UNII: CAL22UVI4M) Ceteth-20 (UNII: I835H2IHHX) Cetyl alcohol (UNII: 936JST6JCN) Menthol (UNII: L7T10EIP3A) Sodium phosphate (UNII: SE337SVY37) Benzalkonium chloride (UNII: F5UM2KM3W7) Tocopherol (UNII: R0ZB2556P8) Lavender Oil (UNII: ZBP1YXW0H8) Aloe Vera Leaf (UNII: ZY81Z83H0X) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43251-3340-1 1 in 1 CARTON 1 56 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333C 08/11/2012 Labeler - LaCorium Health International Pty Ltd (758651624) Establishment Name Address ID/FEI Business Operations Jalco Pharmaceuticals Pty Ltd 757701409 MANUFACTURE(43251-3340) , PACK(43251-3340) , LABEL(43251-3340) , ANALYSIS(43251-3340)