FLEXITOL MEDICATED FOOT- tolnaftate cream
LaCorium Health International Pty Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
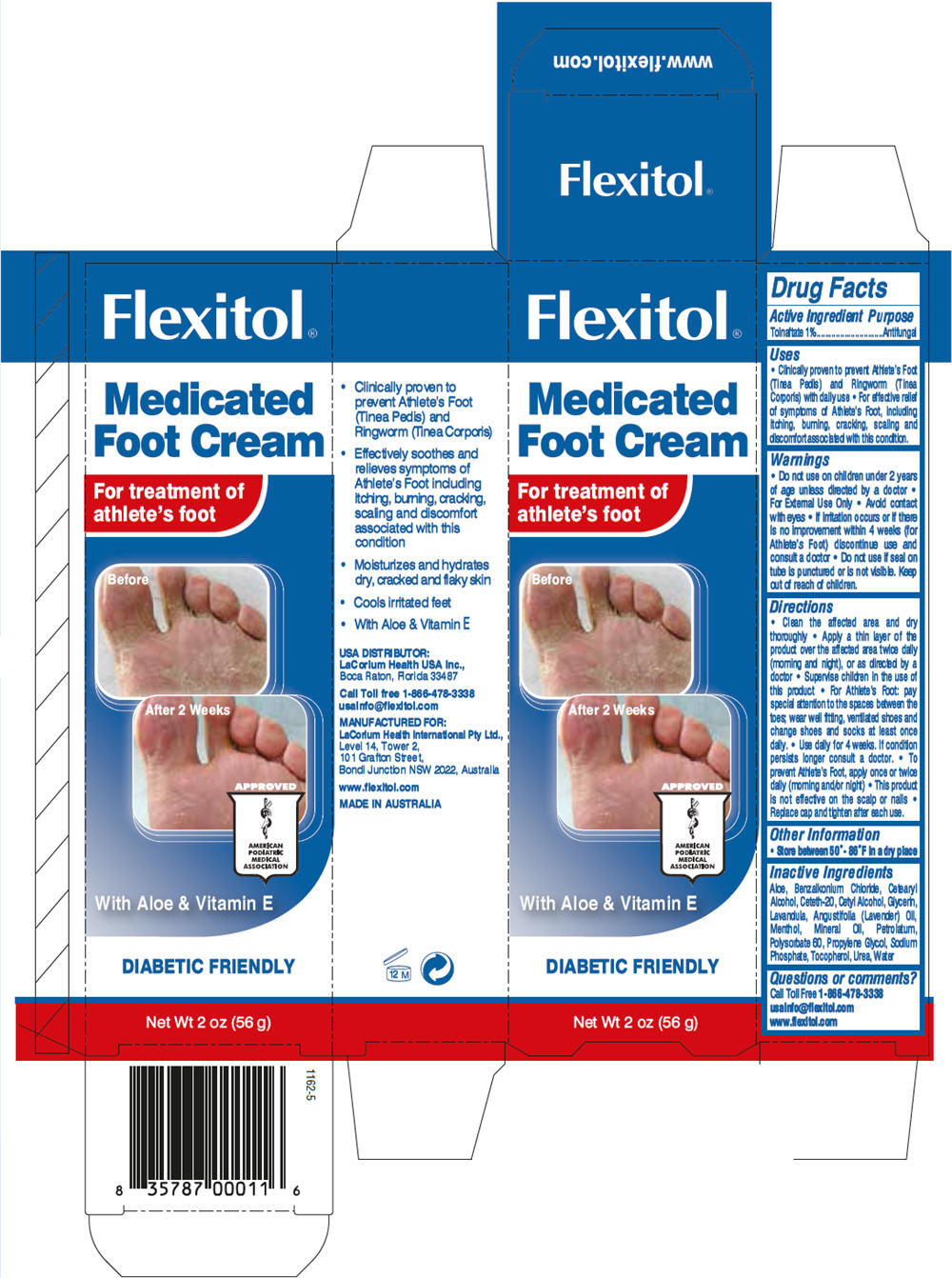
Tolnaftate 1%
Uses
- Clinically proven to prevent Athlete's Foot (Tinea Pedis) and Ringworm (Tinea Corporis) with daily use
- For effective relief of symptoms of Athlete's Foot, including itching, burning, cracking, scaling and discomfort associated with this condition.
Warnings
- Do not use on children under 2 years of age unless directed by a doctor
- For External Use Only
- Avoid contact with eyes
- If irritation occurs or if there is no improvement within 4 weeks (for Athlete's Foot) discontinue use and consult a doctor
- Do not use if seal on tube is punctured or is not visible. Keep out of reach of children.
Directions
- Clean the affected area and dry thoroughly
- Apply a thin layer of the product over the affected area twice daily (morning and night), or as directed by a doctor
- Supervise children in the use of this product
- For Athlete's Foot: pay special attention to the spaces between the toes; wear well fitting, ventilated shoes and change shoes and socks at least once daily.
- Use daily for 4 weeks. If condition persists longer consult a doctor.
- To prevent Athlete's Foot, apply once or twice daily (morning and/or night)
- This product is not effective on the scalp or nails
- Replace cap and tighten after each use.
Other Information
-
Store between 50°- 86°F in a dry place
Inactive Ingredients
Aloe, Benzalkonium Chloride, Cetearyl Alcohol, Ceteth-20, Cetyl Alcohol, Glycerin, Lavandula, Angustifolia (Lavender) Oil, Menthol, Mineral Oil, Petrolatum, Polysorbate 60, Propylene Glycol, Sodium Phosphate, Tocopherol, Urea, Water
Questions or comments?
Call Toll Free 1-866-478-3338
usainfo@flexitol.com
www.flexitol.com
USA DISTRIBUTOR:
LaCorium Health USA Inc.,
Boca Raton, Florida 33487
MANUFACTURED FOR:
LaCorium Health International Pty Ltd.,
Level 14, Tower 2,
101 Grafton Street,
Bondi Junction NSW 2022, Australia
PRINCIPAL DISPLAY PANEL - 56 g Tube Carton
Flexitol®
Medicated
Foot Cream
For treatment of
athlete's foot
Before
After 2 Weeks
APPROVED
AMERICAN
PODIATRIC
MEDICAL
ASSOCIATION
With Aloe & Vitamin E
DIABETIC FRIENDLY
Net Wt 2 oz (56 g)