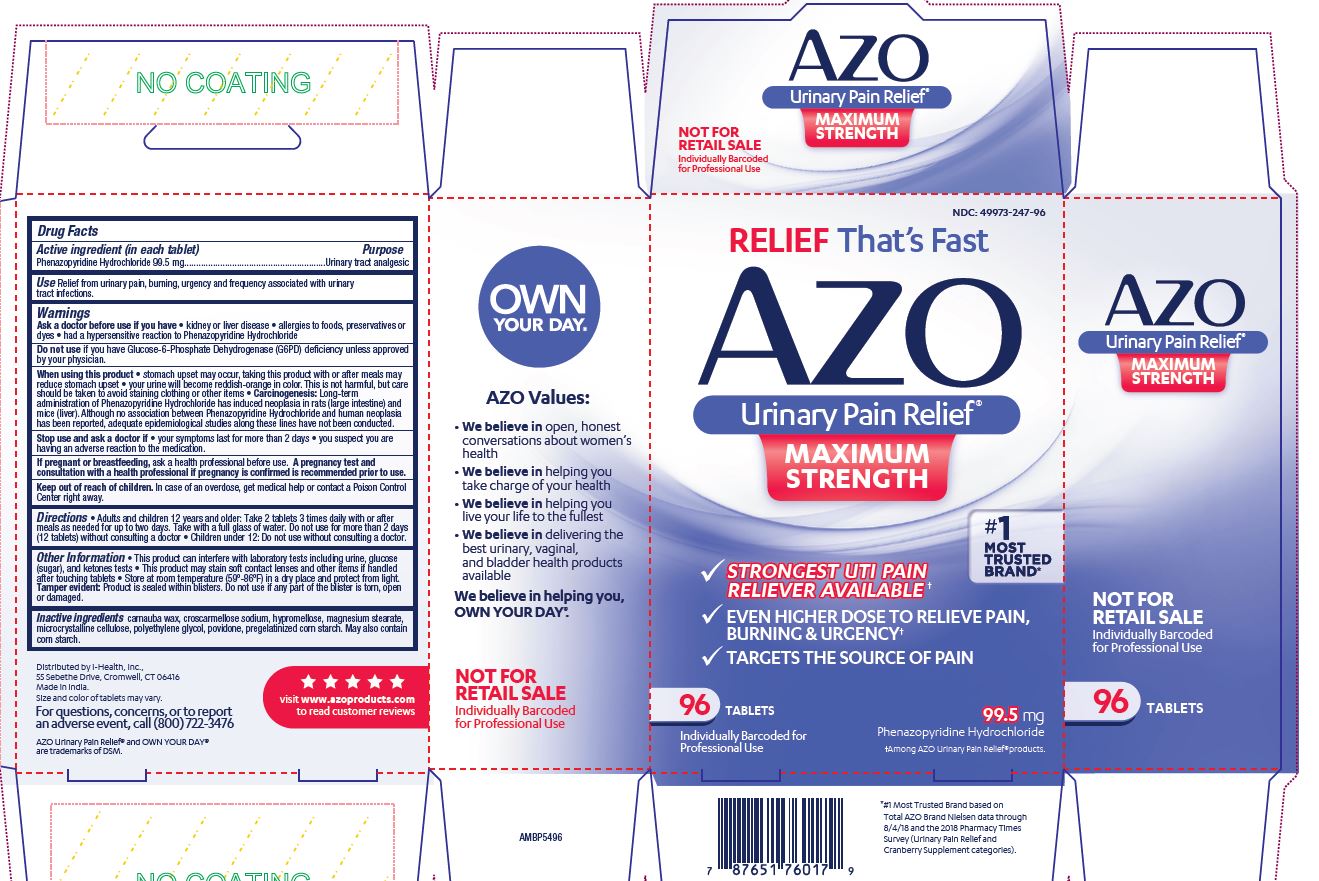
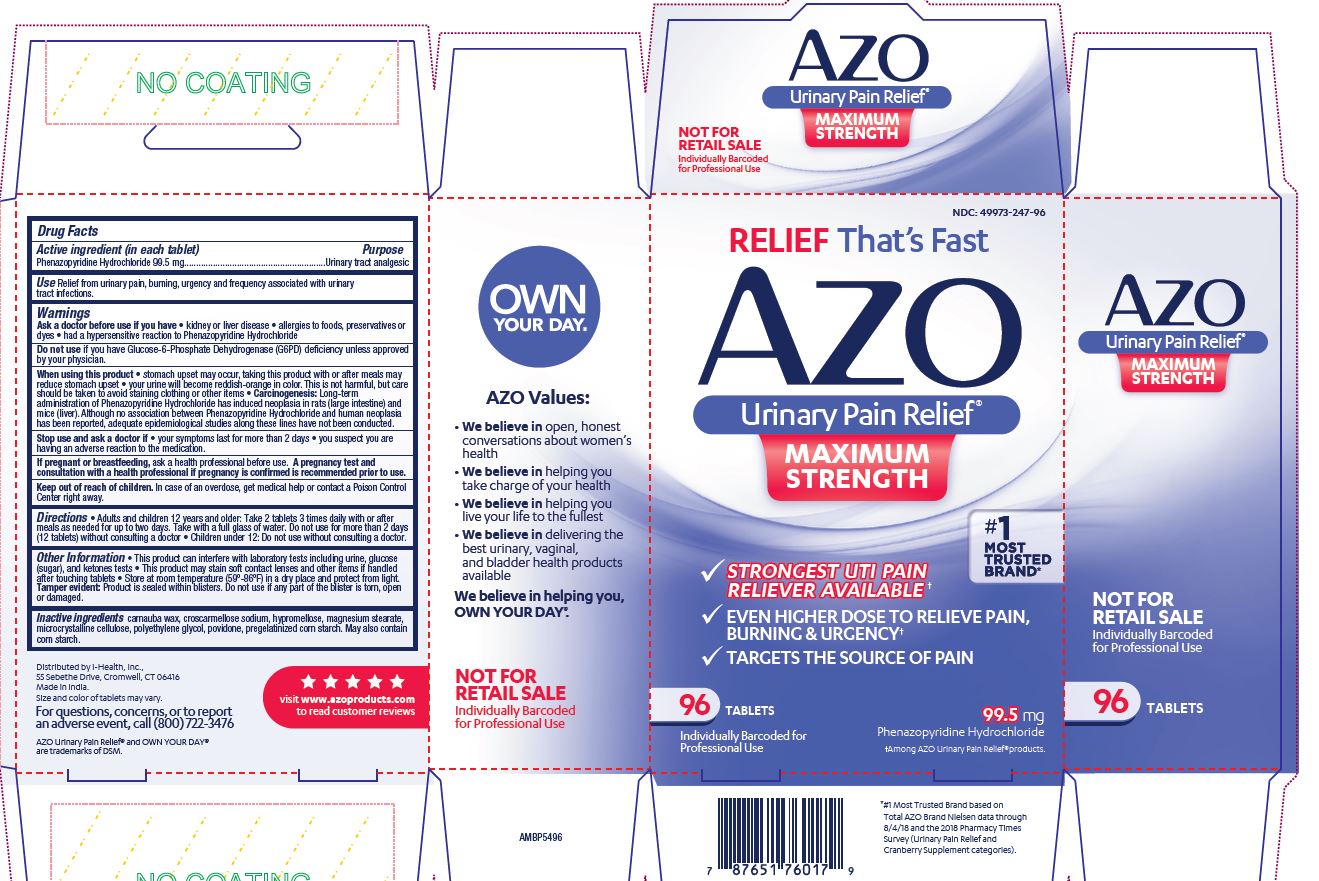
Label: AZO URINARY PAIN RELIEF MAXIMUM STRENGTH- phenazopyridine hydrochloride tablet
- NDC Code(s): 49973-247-06, 49973-247-12, 49973-247-24, 49973-247-96
- Packager: i-Health, Inc.
- This is a repackaged label.
- Source NDC Code(s): 66789-247
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Please read insert for important precautions.
Ask a doctor before use if you have
- kidney disease
- allergies to foods, preservatives or dyes
- had a hypersensitive reaction to Phenazopyridine Hydrochloride
Do not useif you have Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency unless approved by your physician.
When using this product
- stomach upset may occur, taking this product with or after meals may reduce stomach upset
- your urine will become reddish-orange in color. This is not harmful, but care should be taken to avoid staining clothing or other items.
Stop use and ask doctor if
- your symptoms last for more than 2 days
- you suspect you are having an adverse reaction to the medication.
- DOSAGE & ADMINISTRATION
-
OTHER SAFETY INFORMATION
Other Inf ormation
- This product can interfere with laboratory tests including urine, glucose (sugar), and ketones tests
- This product may stain soft contact lenses and other items if handled after touching tablets
- Store at room temperature (59-86 F) in a dry place and protect from light.
Tamper evident: Product is sealed within blisters. Do not use if any part of the blister is torn, open or damaged.
- INACTIVE INGREDIENT
-
SPL UNCLASSIFIED SECTION
Distributed by i-Health, Inc., 55 Sebethe Drive, Cromwell, CT 06416
Made in India. Size and color of tablets may vary.
For questions, concerns, or to report an adverse event, call (800) 722-3476
AZO Urinary Pain Relief ®and OWN YOUR DAY™ are trademarks of DSM.
Información y instrucciones en Español adjuntas
visit www.azoproducts.comto read customer reviews
-
PATIENT PACKAGE INSERT
AZO
Urinary Pain Relief ®
MAXIMUM STRENGTH
Urinary Pain Relief Tablets
Active ingredient: Each analgesic tablet contains Phenazopyridine Hydrochloride 99.5 mg.
Fast relief of urinary discomfort including common urinary tract infection symptoms: Urinary pain, burning, urgency and frequency.
URINARY TRACT INFECTION (UTI)
Symptoms of urinary discomfort may indicate a urinary tract infection. If you experience symptoms such as urinary pain, burning, urgency and frequency, you need to call your doctor to get diagnosed and obtain a prescription to treat the infection. While you wait to get to the doctor and for the prescription to begin to work, you can get fast, symptomatic relief with AZO Urinary Pain Relief Maximum Strength.
WARNINGS
Ask a doctor before use if you have:
- liver or kidney trouble
- allergies to foods, preservatives or dyes
- had a hypersensative reaction to Phenazopyridine Hydrochloride
Do Not Use:
if you have Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency unless approved by your physician.
When using this product
- stomach upset may occur. Taking with or after meals may reduce stomach upset.
- your urine will become reddish-orange in color. This is not harmful, but care should be taken to avoid staining clothing or other items.
Stop using ths product and ask a doctorif you think you may be having an adverse reaction to the medication or if your symptoms last for more than two days.
If you are pregnant or breastfeeding, ask a healthcare professional before you use this product. A pregnancy test and consultation with a health professional if pregnancy is confirmed is recommended prior to use.
Keep this medication out of the reach of children.
In case of an overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Adults and children 12 years and older: Take two (2) tablets three (3) times daily for up to two days. Take with a full glass of water, with or after meals as needed. Do not use for more than two days (12 tablets) without consulting a doctor.
Children under age 12: do not use without consulting a doctor.
OTHER INFORMATION & PRECAUTIONS
- This product can interfere with laboratory tests including urine, glucose (sugar) and keytones.
- This product may stain soft contact lenses and other items if handled after touching tablets.
- Size and color of tablets may vary.
- Carcinogenesis: Long-term administration of Phenazopyridine Hydrochloride has induced neoplasia in rats (large intestine) and mice (liver). Although no association between Phenazopyridine Hydrochloride and human neoplasia has been reported, adequate epidemiological studies along these lines have not been conducted.
- Storage: Store at room temperature (59° - 86°F) in a dry place and protect from light.
- Tamper Evident: Product is sealed within blisters. Do not use if any part of the blister is torn, open or damanged.
For questions, concerns, or to report an adverse event, please call (800) 722-3476 or visit www.azoproducts.com
AZO
Urinary Pain Relief ®
MAXIMUM STRENGTH
Alivio rápido para el dolor de las vías urinarias
Ingrediente activo: Cada tableta de analgésico contiene 99.5 mg
de hidrocloruro de fenazopiridina.
Alivio rápido de la incomodidad en las vías urinarias que incluye
los síntomas comunes de infección en las vías urinarias: dolor al
orinar, ardor, urgencia y frecuencia.
INFECCIÓN DE LAS VÍAS URINARIAS
Los síntomas de incomodidad en las vías urinarias pueden
indicar la presencia de una infección. Si tiene síntomas como
dolor, ardor, urgencia y frecuencia, necesita llamar a su médico
para recibir un diagnóstico y que le dé una receta para tratar la
infección. Mientras espera para ver al doctor y para que el
medicamento que le recete comience a funcionar, puede
obtener un alivio rápido de sus síntomas con AZO Urinary Pain
Relief® Maximum Strength.
ADVERTENCIAS
Consulte a su médico antes de usar este producto si:
•tiene problemas renales o hepáticos.
•tiene alergias a alimentos, conservadores o tintes.
•ha tenido una reacción alérgica a el hidrocloruro de
fenazopiridina.
No usar: si tiene una deficiencia de glucosa-6-fosfato
deshidrogenasa (G6PD), a menos que su médico lo apruebe.
Al usar este producto:
•puede sentir malestar estomacal. Tómelo durante o después
de las comidas para ayudar a disminuir el malestar.
• la orina adquiere un color anaranjado rojizo.
Esto no es dañino, pero debe tener cuidado para evitar
manchar a ropa u otros artículos.
Deje de usar este producto y consulte a su médicosi piensa
que puede estar teniendo una reacción adversa al medicamento
o si sus síntomas duran más de dos días.
Si usted está embarazada o lactando, consulte a un profesional
médico antes de usar este producto. Se recomienda una prueba
de embarazo y una consulta con un profesional médico si se
confirma un embarazo antes de usar el producto.
Mantenga este medicamento fuera del alcance de los niños.
En caso de una sobredosis, consiga atención médica o
comuníquese con un centro de control de envenenamiento de
inmediato.
INSTRUCCIONES
Adultos y niños mayores de 12 años: tome dos (2) tabletas tres
(3) veces al día hasta dos días. Tome con un vaso de agua, durante
o después de las comidas, según sea necesario. No lo utilice
durante más de 2 días (12 tabletas) sin consultar a un médico.
Niños menores de 12 años: no se utilice sin consultar a un médico.
OTRA INFORMACIÓN y PRECAUCIONES
•Este producto puede interferir con los análisis de
laboratorio, incluso los análisis de orina, glucosa (azúcar)
y cetonas.
•Este producto puede manchar los lentes de contacto blandos
y otros artículos si los manipula después de tocar las tabletas.
•El tamaño y el color de las tabletas puede variar.
• Carcinogénesis: la administración a largo plazo del
hidrocloruro de fenazopiridina ha inducido neoplasia en
ratas (intestino grueso) y ratones (hígado). Aunque no se ha
informado acerca de ningún caso que relacione el
hidrocloruro de fenazopiridina con la neoplasia en
humanos, no se han efectuado estudios epidemiológicos
adecuados al respecto.
• Almacenaje: almacénese a temperatura ambiental
(15° a 30°C) en un sitio seco, y protéjase de la luz.
•Evidencia de alteraciones: el producto viene sellado en
blísteres. No lo use si alguna parte del blíster está rota,
abierta o dañada.
Para preguntas, dudas o para informar acerca de una reacción adversa,
llame al (800) 722-3476 o visite www.azoproducts.com
-
Packaging
RELIEF That's Fast
AZO
Urinary Pain Relief®
MAXIMUM STRENGTH
- STRONGEST UTI PAIN RELIEVER AVAILABLE+
- EVEN HIGHER DOSE TO RELEIVE PAIN, BURNING & URGENCY+
- TARGETS THE SOURCE OF PAIN
#1 MOST TRUSTED BRAND*
__ TABLETS
99.5 mg
Phenazopyridine Hydrochloride
+Among AZO Urinary Pain Relief® products.
* #1 Most Trusted Brand based on Total AZO Brand Nielsen data through 1/20/18 and the 2017 Pharmacy Times Survey (Urinary Pain Relief and Cranberry Supplement categories).







-
INGREDIENTS AND APPEARANCE
AZO URINARY PAIN RELIEF MAXIMUM STRENGTH
phenazopyridine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49973-247(NDC:66789-247) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENAZOPYRIDINE HYDROCHLORIDE (UNII: 0EWG668W17) (PHENAZOPYRIDINE - UNII:K2J09EMJ52) PHENAZOPYRIDINE HYDROCHLORIDE 99.5 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) CARNAUBA WAX (UNII: R12CBM0EIZ) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color red (Maroon) Score no score Shape CAPSULE Size 9mm Flavor Imprint Code 99 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49973-247-12 6 in 1 BOX 07/02/2018 1 2 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:49973-247-24 12 in 1 BOX 07/02/2018 2 2 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:49973-247-96 48 in 1 BOX 02/22/2019 01/31/2023 3 2 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:49973-247-06 3 in 1 BOX 06/15/2023 4 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/02/2018 Labeler - i-Health, Inc. (061427694)