Label: VAGISTEN-V 7 DAY- miconazole nitrate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 69729-612-01, 69729-612-05 - Packager: OPMX LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 6, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
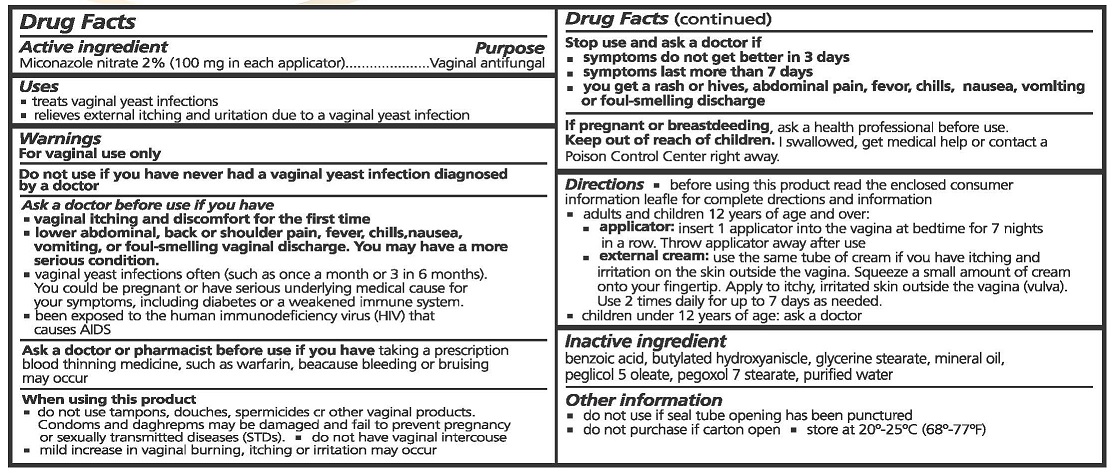
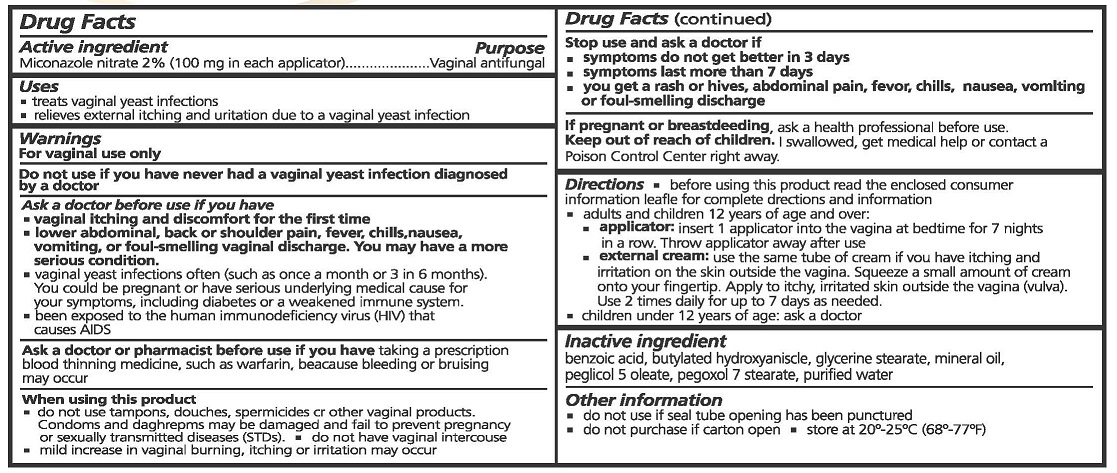
- ACTIVE INGREDIENTS
- PURPOSE
- USES
-
WARNINGS
FOR VAGINAL USE ONLY.
DO NOT USE IF YOU HAVE NEVER HAD A VAGINAL YEAST INFECTION DIAGNOSED BY A DOCTOR.
ASK A DOCTOR BEFORE USE IF YOU HAVE
- VAGINAL ITCHING AND DISCOMFORT FOR FIRST TIME
- LOWER ABDOMINAL, BACK OR SHOULDER PAIN, FEVER, CHILLS, NASEA, VOMITING, OR FOUL-SMELLING VAGINAL DISCHARGE. YOU MAY HAVE A MORE SERIOUS CONDITION.
- VAGINAL YEAST INFECTIONS OFTEN (SUCH AS ONCE A MONTH OR 3 IN 6 MONTHS). YOU COULD BE PREGNANT OR HAVE SERIOUS UNDERLYING MEDICAL CAUSE FOR YOUR SYMPTOMS, INCLUDING DIABETES OR A WEAKENED IMMUNE SYSTEM.
- BEEN EXPOSED TO THE HUMAN IMMUNODEFICIENCY VIRUS (HIV) THAT CAUSES AIDS.
ASK A DOCTOR OR PHARMACIST BEFORE USE IF YOU HAVE TAKEN A PRESCRIPTION BLOOD THINNING MEDICINE, SUCH AS WARFARIN, BECAUSE BLEEDING OR BRUISING MAY OCCUR.
WHEN USING THIS PRODUCT
- DO NOT USE TAMPONS, DOUCHES, SPERMACIDES OR OTHER VAGINAL PRODUCTS. CONDOMS AND DIAPHRAGMS MAY BE DAMAGED OR FAIL TO PREVENT PREGNANCY OR SEXUALLY TRANSMITTED DISEASES (STDS).
- DO NOT HAVE VAGINAL INTERCOURSE
- MILD INCREASE IN VAGINAL BURNING, ITCHING OR IRRITATION MAY OCCUR.
STOP USE AND ASK A DOCTOR IF
- SYMPTOMS DO NOT GET BETTER IN 3 DAYS
- SYMPTOMS LAST MORE THAN 7 DAYS
- YOU GET A RASH OR HIVESS, ABDOMINAL PAIN, FEVER, CHILLS, NAUSEA, VOMITING OR FOUL-SMELLING DISCHARGE
IF PREGNANT OR BREAST FEEDING, ASK A HEALTH PROFESSIONAL BEFORE USE.
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- BEFORE USING THIS PRODUCT READ THE ENCLOSED CONSUMER INFORMATION LEAFLET FOR COMPLETE DIRECTIONS AND INFORMATION
- ADULTS AND CHILDREN 12 YEARS OF AGE OR OVER:
- APPLICATOR: INSERT 1 APPLICATOR INTO THE VAGINA AT BEDTIME FOR 7 NIGHTS IN A ROW. THROW APPLICATOR AWAY AFTER USE.
- EXTERNAL CREAM: USE THE SAME TUBE OF CREAM IF YOU HAVE ITCHING AND IRRITATION ON THE SKIN OUTSIDE THE VAGINA. SQUEEZE A SMALL AMOUNT OF CREAM ONTO YOUR FINGERTIP. APPLY TO ITCHY, IRRITATED SKIN OUTSIDE THE VAGINA (VULVA). USE 2 TIMES DAILY FOR UP TO 7 DAYS AS NEEDED.
- CHILDREN UNDER 12 YEARS OF AGE: ASK A DOCTOR.
- INACTIVE INGREDIENTS
- OTHER INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VAGISTEN-V 7 DAY
miconazole nitrate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69729-612 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 2 g in 100 g Inactive Ingredients Ingredient Name Strength BENZOIC ACID (UNII: 8SKN0B0MIM) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MINERAL OIL (UNII: T5L8T28FGP) PEG-5 OLEATE (UNII: 0240V77G50) PEGOXOL 7 STEARATE (UNII: 3EW5AXE5X5) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69729-612-05 1 in 1 BOX 09/05/2018 1 NDC:69729-612-01 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 09/05/2018 Labeler - OPMX LLC (029918743)