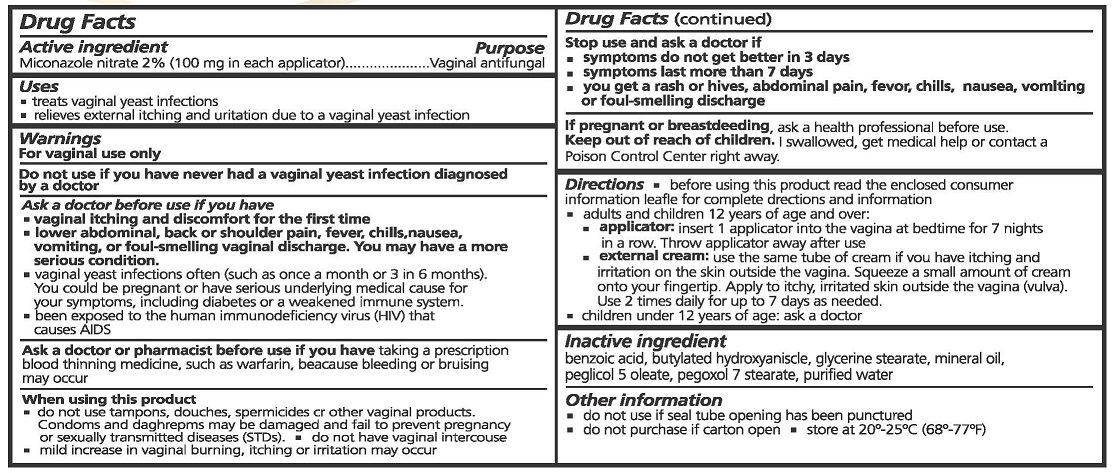
USES
- TREATS VAGINAL YEAST INFECTIONS
- RELIEVES EXTERNAL ITCHING AND URINATION DUE TO VAGINAL YEAST INFECTION
WARNINGS
FOR VAGINAL USE ONLY.
DO NOT USE IF YOU HAVE NEVER HAD A VAGINAL YEAST INFECTION DIAGNOSED BY A DOCTOR.
ASK A DOCTOR BEFORE USE IF YOU HAVE
- VAGINAL ITCHING AND DISCOMFORT FOR FIRST TIME
- LOWER ABDOMINAL, BACK OR SHOULDER PAIN, FEVER, CHILLS, NASEA, VOMITING, OR FOUL-SMELLING VAGINAL DISCHARGE. YOU MAY HAVE A MORE SERIOUS CONDITION.
- VAGINAL YEAST INFECTIONS OFTEN (SUCH AS ONCE A MONTH OR 3 IN 6 MONTHS). YOU COULD BE PREGNANT OR HAVE SERIOUS UNDERLYING MEDICAL CAUSE FOR YOUR SYMPTOMS, INCLUDING DIABETES OR A WEAKENED IMMUNE SYSTEM.
- BEEN EXPOSED TO THE HUMAN IMMUNODEFICIENCY VIRUS (HIV) THAT CAUSES AIDS.
ASK A DOCTOR OR PHARMACIST BEFORE USE IF YOU HAVE TAKEN A PRESCRIPTION BLOOD THINNING MEDICINE, SUCH AS WARFARIN, BECAUSE BLEEDING OR BRUISING MAY OCCUR.
WHEN USING THIS PRODUCT
- DO NOT USE TAMPONS, DOUCHES, SPERMACIDES OR OTHER VAGINAL PRODUCTS. CONDOMS AND DIAPHRAGMS MAY BE DAMAGED OR FAIL TO PREVENT PREGNANCY OR SEXUALLY TRANSMITTED DISEASES (STDS).
- DO NOT HAVE VAGINAL INTERCOURSE
- MILD INCREASE IN VAGINAL BURNING, ITCHING OR IRRITATION MAY OCCUR.
STOP USE AND ASK A DOCTOR IF
- SYMPTOMS DO NOT GET BETTER IN 3 DAYS
- SYMPTOMS LAST MORE THAN 7 DAYS
- YOU GET A RASH OR HIVESS, ABDOMINAL PAIN, FEVER, CHILLS, NAUSEA, VOMITING OR FOUL-SMELLING DISCHARGE
IF PREGNANT OR BREAST FEEDING, ASK A HEALTH PROFESSIONAL BEFORE USE.
Keep out of reach of children
- If swallowed get medical help or contact Poison Control Center right away.
DIRECTIONS
- BEFORE USING THIS PRODUCT READ THE ENCLOSED CONSUMER INFORMATION LEAFLET FOR COMPLETE DIRECTIONS AND INFORMATION
- ADULTS AND CHILDREN 12 YEARS OF AGE OR OVER:
- APPLICATOR: INSERT 1 APPLICATOR INTO THE VAGINA AT BEDTIME FOR 7 NIGHTS IN A ROW. THROW APPLICATOR AWAY AFTER USE.
- EXTERNAL CREAM: USE THE SAME TUBE OF CREAM IF YOU HAVE ITCHING AND IRRITATION ON THE SKIN OUTSIDE THE VAGINA. SQUEEZE A SMALL AMOUNT OF CREAM ONTO YOUR FINGERTIP. APPLY TO ITCHY, IRRITATED SKIN OUTSIDE THE VAGINA (VULVA). USE 2 TIMES DAILY FOR UP TO 7 DAYS AS NEEDED.
- CHILDREN UNDER 12 YEARS OF AGE: ASK A DOCTOR.
INACTIVE INGREDIENTS
BENZOIC ACID, BUTYLATED HYDROXYANISOLE, GLYCERINE STEARATE, MINERAL OIL, PEGLICOL 5 OLEATE, PEGOXOL 7 STEARATE, PURIFIED WATER