Label: QUIK-CARE- ethyl alcohol solution
-
NDC Code(s):
47593-491-38,
47593-491-41,
47593-491-55,
47593-491-56, view more47593-491-57, 47593-491-85
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
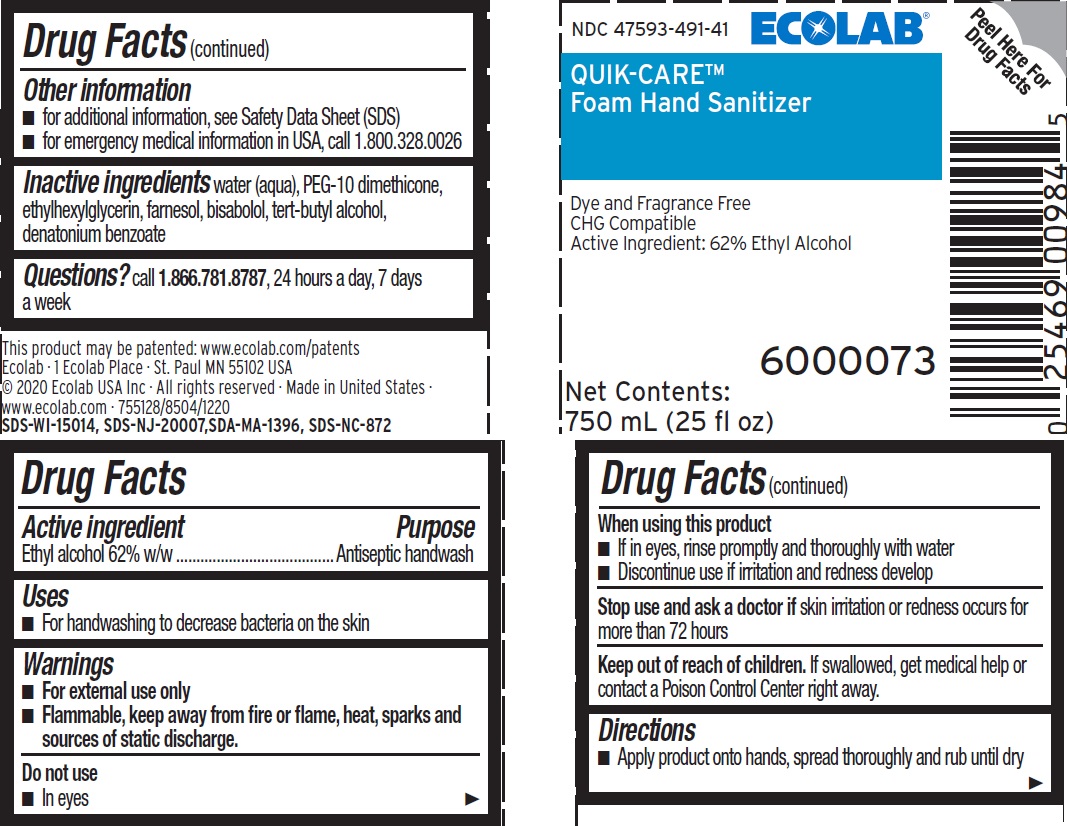
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- QUESTIONS
-
Principal display panel and representative label
NDC 47593-491-41
ECOLAB®
QUIK-CARE™
Foam Hand Sanitizer
Dye and Fragrance Free
CHG Compatible
Active Ingredient: 62% Ethyl Alcohol
Net Contents: 750mL (25 fl oz)
6000073
This product may be patented: www.ecolab.com/patents
Ecolab · 1 Ecolab Place · St Paul MN 55102 USA
© 2020 Ecolab USA Inc · All rights reserved
Made in United States
www.ecolab.com - 755128/8504/1220
SDS-WI-15014, SDS-NJ-20007, SDA-MA-1396, SDS-NC-872
-
INGREDIENTS AND APPEARANCE
QUIK-CARE
ethyl alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-491 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Alcohol (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) Alcohol 539.71 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) FARNESOL (UNII: EB41QIU6JL) .ALPHA.-BISABOLOL, (+/-)- (UNII: 36HQN158VC) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-491-55 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/19/2013 2 NDC:47593-491-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/19/2013 3 NDC:47593-491-38 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/19/2013 12/20/2023 4 NDC:47593-491-85 45 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/29/2014 5 NDC:47593-491-56 1200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/19/2013 6 NDC:47593-491-57 535 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/09/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 06/19/2013 Labeler - Ecolab Inc. (006154611)