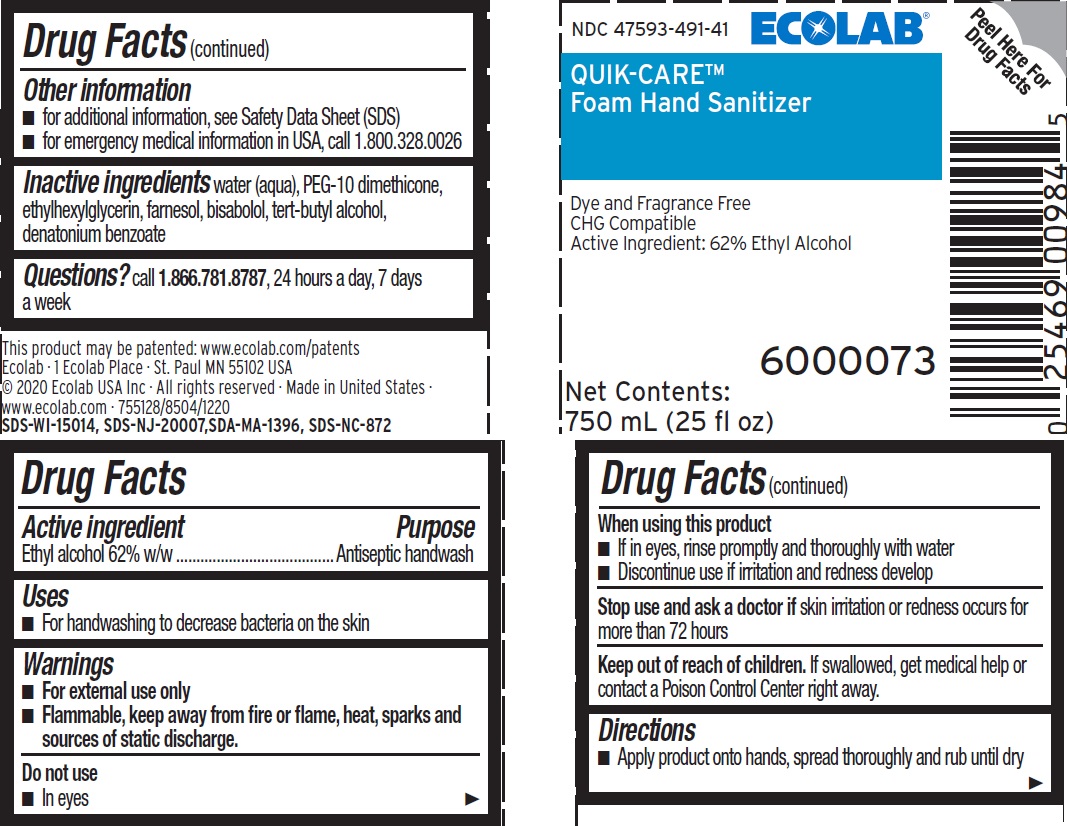
Label: QUIK-CARE- ethyl alcohol solution
-
NDC Code(s):
47593-491-38,
47593-491-41,
47593-491-55,
47593-491-56, view more47593-491-57, 47593-491-85
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 26, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- QUESTIONS
-
Principal display panel and representative label
NDC 47593-491-41
ECOLAB®
QUIK-CARE™
Foam Hand Sanitizer
Dye and Fragrance Free
CHG Compatible
Active Ingredient: 62% Ethyl Alcohol
Net Contents: 750mL (25 fl oz)
6000073
This product may be patented: www.ecolab.com/patents
Ecolab · 1 Ecolab Place · St Paul MN 55102 USA
© 2020 Ecolab USA Inc · All rights reserved
Made in United States
www.ecolab.com - 755128/8504/1220
SDS-WI-15014, SDS-NJ-20007, SDA-MA-1396, SDS-NC-872
-
INGREDIENTS AND APPEARANCE
QUIK-CARE
ethyl alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-491 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Alcohol (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) Alcohol 539.71 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) FARNESOL (UNII: EB41QIU6JL) .ALPHA.-BISABOLOL, (+/-)- (UNII: 36HQN158VC) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-491-55 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/19/2013 2 NDC:47593-491-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/19/2013 3 NDC:47593-491-38 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/19/2013 12/20/2023 4 NDC:47593-491-85 45 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/29/2014 5 NDC:47593-491-56 1200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/19/2013 6 NDC:47593-491-57 535 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/09/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/19/2013 Labeler - Ecolab Inc. (006154611)