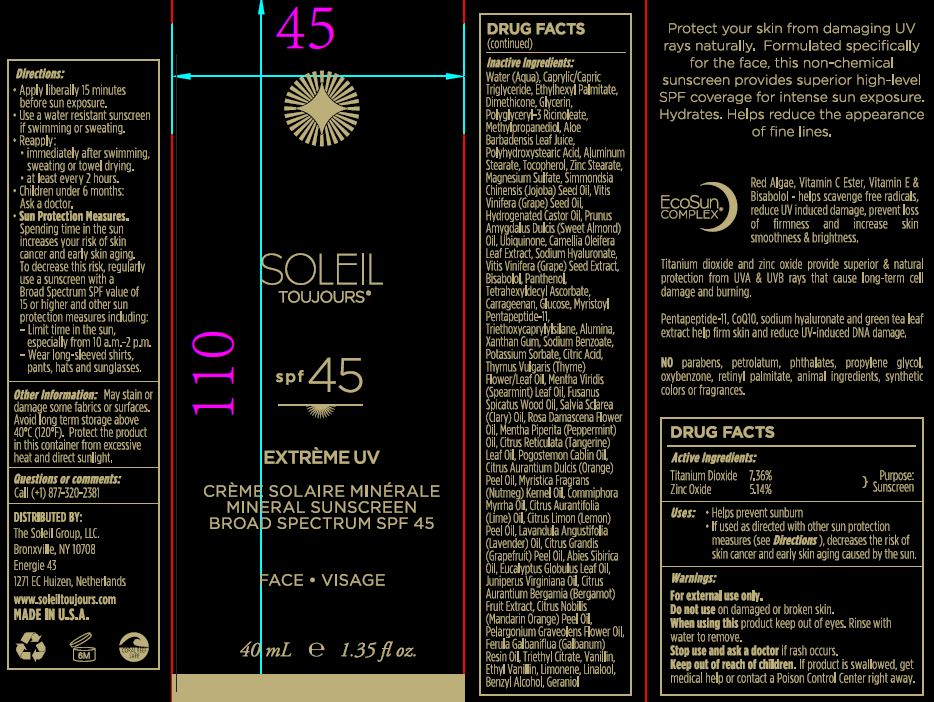
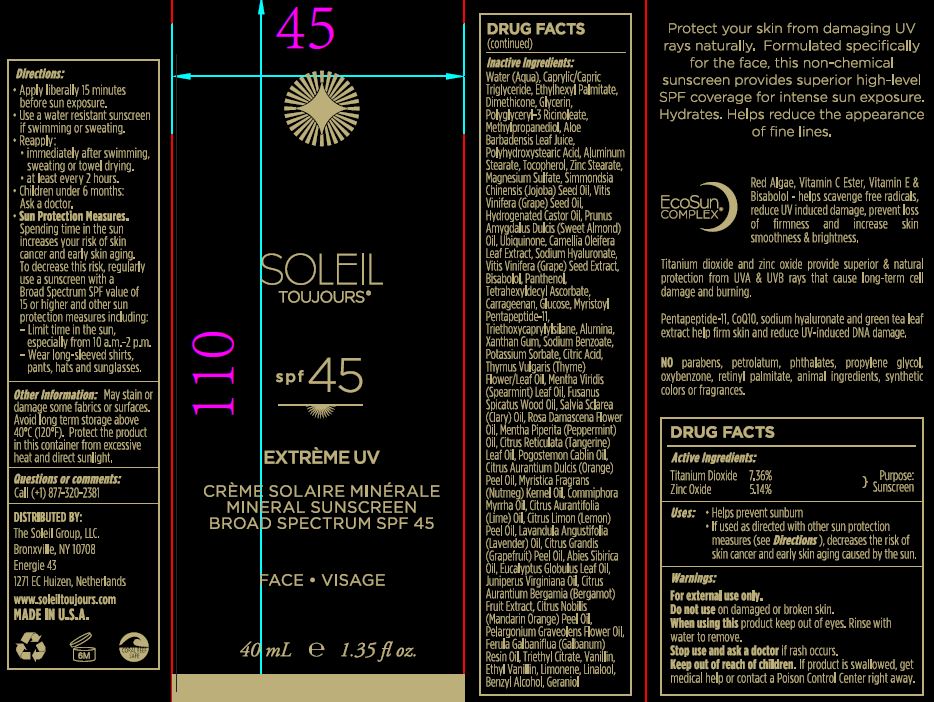
Label: MINERAL SUNSCREEN BROAD SPECTRUM SPF 45- zinc oxide and titanium dioxide cream
- NDC Code(s): 62742-4152-1, 62742-4152-2
- Packager: Allure Labs, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 11, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
apply liberally 15 minutes before sun exposure.
use a water resistant sunscreen if swimming or sweating.
reapply:
immidiately aftr swimming, sweating or towel drying.
at least every 2 hours.
children under 6months: ask doctor
Sun protection measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protector measures including:
limit time in the sun, especially from 10:00 am to 2:00 pm.
wear long sleaved shirts, pants, hats and sun glasses.
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Water (Aqua), Caprylic/Capric Triglyceride, Ethylhexyl Palmitate, Dimethicone, Glycerin, Polyglyceryl-3 Ricinoleate, Methylpropanediol, Aloe Barbadensis Leaf Juice, Polyhydroxystearic Acid, Aluminum Stearate, Tocopherol, Zinc Stearare, Magnesium Sulfate, Simmondsia Chinesis (Jojoba) Seed Oil, Vitis Vinifera (Grape) Seed Oil, Hydrogenated Castor oil, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Ubiquinone, Camellia Oleifera Leaf Extract, Sodium Hyaluronate, Vitis Vinifera (Grape) Seed Extract, Bisabolol, Panthenol, Tetrahexyldecyl Ascorbate, Carrageenan, Glucose, Myristoyl Pentapeptide-11, Triethoxycaprylylsilane, Alumina, Xanthan Gum, Sodium Benzoate, Potassium Sorbate, Citric Acid, Thymus Vulgaris (Thyme) Flower/Leaf Oil, Mentha Virdis (Spearmint) Leaf Oil, Fusanus Spicatus Wood Oil, Salvia Sclarea (Clary) Oil, Rosa Damascena Flower Oil, Mentha Piperita (Peppermint) Oil, Citrus Reticulata (Tangerine) Leaf Oil, Pogostemon Cablin Oil, Citrus Aurantium Dulcis (Orange) Peel Oil, Myritica Fragrans (Nutmeg) Kernel Oil, Commiphora Myrrha Oil, Citrus Aurantifolia (Lime) Oil, Citrus Limon (Lemon) Peel Oil, Lavandula Angustifolia (Lavender) Oil, Citrus Grandis (Grapefruit) Peel oil, Abies Sibirica Oil, Eucalyptus Globulus Leaf Oil, Juniperus Virginiana Oil, Citrus Aurantium Bergamia (Bergamot) Fruit Extract, Citrus Nobilis (Mandarin Orange) Peel Oil, Pelargonium Graveolens Flower Oil, Ferula Galbaniflua (Galbanum) Resin Oil, Triethyl Citrate, Vanillin, ethyl Vanillin, Limonene, Linalool, benzyl Alcohol, Geraniol.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINERAL SUNSCREEN BROAD SPECTRUM SPF 45
zinc oxide and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62742-4152 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 51.4 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 73.6 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ETHYLHEXYL PALMITATE (UNII: 2865993309) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) METHYLPROPANEDIOL (UNII: N8F53B3R4R) aloe (UNII: V5VD430YW9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TOCOPHEROL (UNII: R0ZB2556P8) ZINC STEARATE (UNII: H92E6QA4FV) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) SIMMONDSIA CHINENSIS SEED (UNII: D24K2Q1F6H) VITIS VINIFERA SEED (UNII: C34U15ICXA) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) ALMOND OIL (UNII: 66YXD4DKO9) UBIQUINONE Q2 (UNII: I7T5V2W47R) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) PANTHENOL (UNII: WV9CM0O67Z) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) CARRAGEENAN (UNII: 5C69YCD2YJ) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALUMINUM OXIDE (UNII: LMI26O6933) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) THYMUS VULGARIS LEAF (UNII: GRX3499643) SPEARMINT OIL (UNII: C3M81465G5) CLARY SAGE OIL (UNII: 87L0D4U3M0) SANTALUM SPICATUM OIL (UNII: H9LVS6REV4) ROSA DAMASCENA FLOWER OIL (UNII: 18920M3T13) PEPPERMINT OIL (UNII: AV092KU4JH) CITRUS RETICULATA LEAF OIL (UNII: 1515UE78IH) PATCHOULI OIL (UNII: F3IN55X5PO) ORANGE OIL (UNII: AKN3KSD11B) MYRRH OIL (UNII: H74221J5J4) LIME OIL (UNII: UZH29XGA8G) LEMON OIL (UNII: I9GRO824LL) LAVENDER OIL (UNII: ZBP1YXW0H8) GRAPEFRUIT OIL (UNII: YR377U58W9) ABIES SIBIRICA LEAF OIL (UNII: XRY0V4VZKZ) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) JUNIPERUS VIRGINIANA OIL (UNII: PAD4FN7P2G) BERGAMOT OIL (UNII: 39W1PKE3JI) TANGERINE (UNII: KH3E3096OO) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) GALBANUM OIL (UNII: 211UF7M8N1) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) VANILLIN (UNII: CHI530446X) ETHYL VANILLIN (UNII: YC9ST449YJ) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+)- (UNII: F4VNO44C09) BENZYL ALCOHOL (UNII: LKG8494WBH) GERANIOL (UNII: L837108USY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62742-4152-2 1 in 1 CARTON 12/11/2017 1 NDC:62742-4152-1 40 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 12/11/2017 Labeler - Allure Labs, Inc (926831603) Registrant - Allure Labs, Inc (926831603) Establishment Name Address ID/FEI Business Operations Allure Labs, Inc 926831603 manufacture(62742-4152)