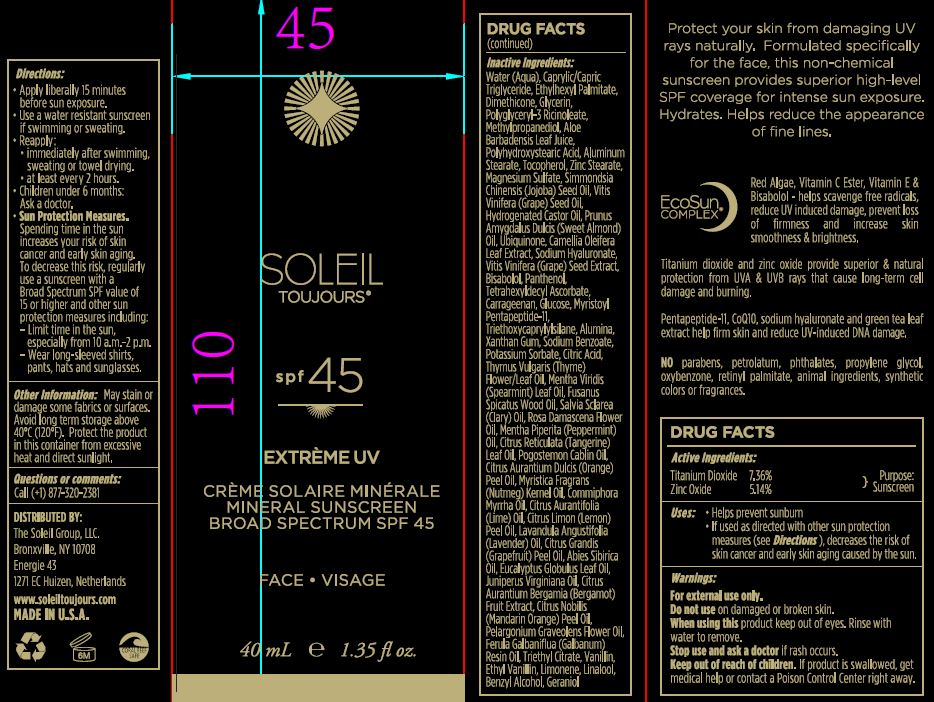
Helps prevent sunburn
if used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.
apply liberally 15 minutes before sun exposure.
use a water resistant sunscreen if swimming or sweating.
reapply:
immidiately aftr swimming, sweating or towel drying.
at least every 2 hours.
children under 6months: ask doctor
Sun protection measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protector measures including:
limit time in the sun, especially from 10:00 am to 2:00 pm.
wear long sleaved shirts, pants, hats and sun glasses.
May stain or damage some fabrics or surfaces. Avoid long term storage above 40C (120F). Protect the product in this container from excessive heat and direct sunlight.
Water (Aqua), Caprylic/Capric Triglyceride, Ethylhexyl Palmitate, Dimethicone, Glycerin, Polyglyceryl-3 Ricinoleate, Methylpropanediol, Aloe Barbadensis Leaf Juice, Polyhydroxystearic Acid, Aluminum Stearate, Tocopherol, Zinc Stearare, Magnesium Sulfate, Simmondsia Chinesis (Jojoba) Seed Oil, Vitis Vinifera (Grape) Seed Oil, Hydrogenated Castor oil, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Ubiquinone, Camellia Oleifera Leaf Extract, Sodium Hyaluronate, Vitis Vinifera (Grape) Seed Extract, Bisabolol, Panthenol, Tetrahexyldecyl Ascorbate, Carrageenan, Glucose, Myristoyl Pentapeptide-11, Triethoxycaprylylsilane, Alumina, Xanthan Gum, Sodium Benzoate, Potassium Sorbate, Citric Acid, Thymus Vulgaris (Thyme) Flower/Leaf Oil, Mentha Virdis (Spearmint) Leaf Oil, Fusanus Spicatus Wood Oil, Salvia Sclarea (Clary) Oil, Rosa Damascena Flower Oil, Mentha Piperita (Peppermint) Oil, Citrus Reticulata (Tangerine) Leaf Oil, Pogostemon Cablin Oil, Citrus Aurantium Dulcis (Orange) Peel Oil, Myritica Fragrans (Nutmeg) Kernel Oil, Commiphora Myrrha Oil, Citrus Aurantifolia (Lime) Oil, Citrus Limon (Lemon) Peel Oil, Lavandula Angustifolia (Lavender) Oil, Citrus Grandis (Grapefruit) Peel oil, Abies Sibirica Oil, Eucalyptus Globulus Leaf Oil, Juniperus Virginiana Oil, Citrus Aurantium Bergamia (Bergamot) Fruit Extract, Citrus Nobilis (Mandarin Orange) Peel Oil, Pelargonium Graveolens Flower Oil, Ferula Galbaniflua (Galbanum) Resin Oil, Triethyl Citrate, Vanillin, ethyl Vanillin, Limonene, Linalool, benzyl Alcohol, Geraniol.