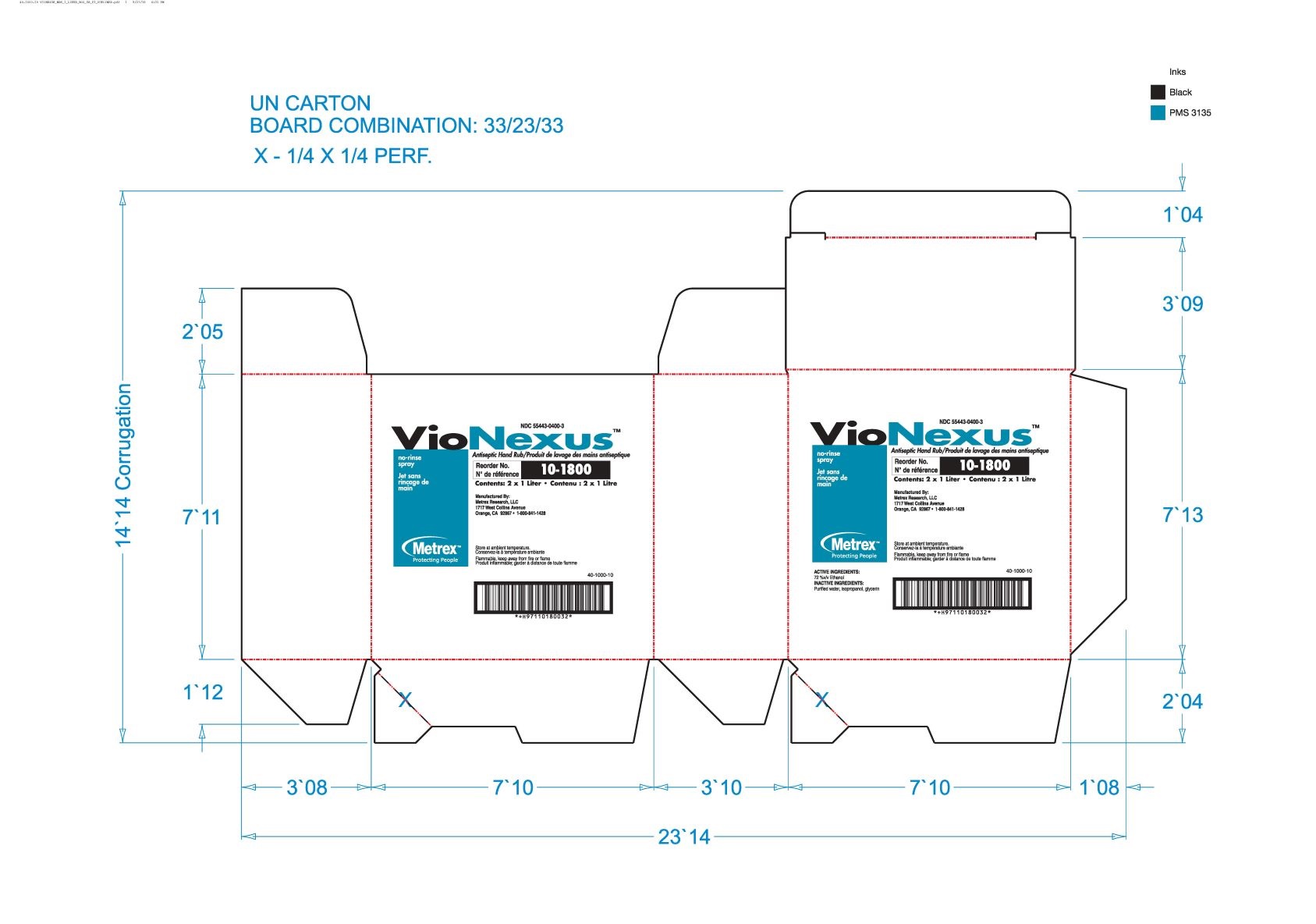
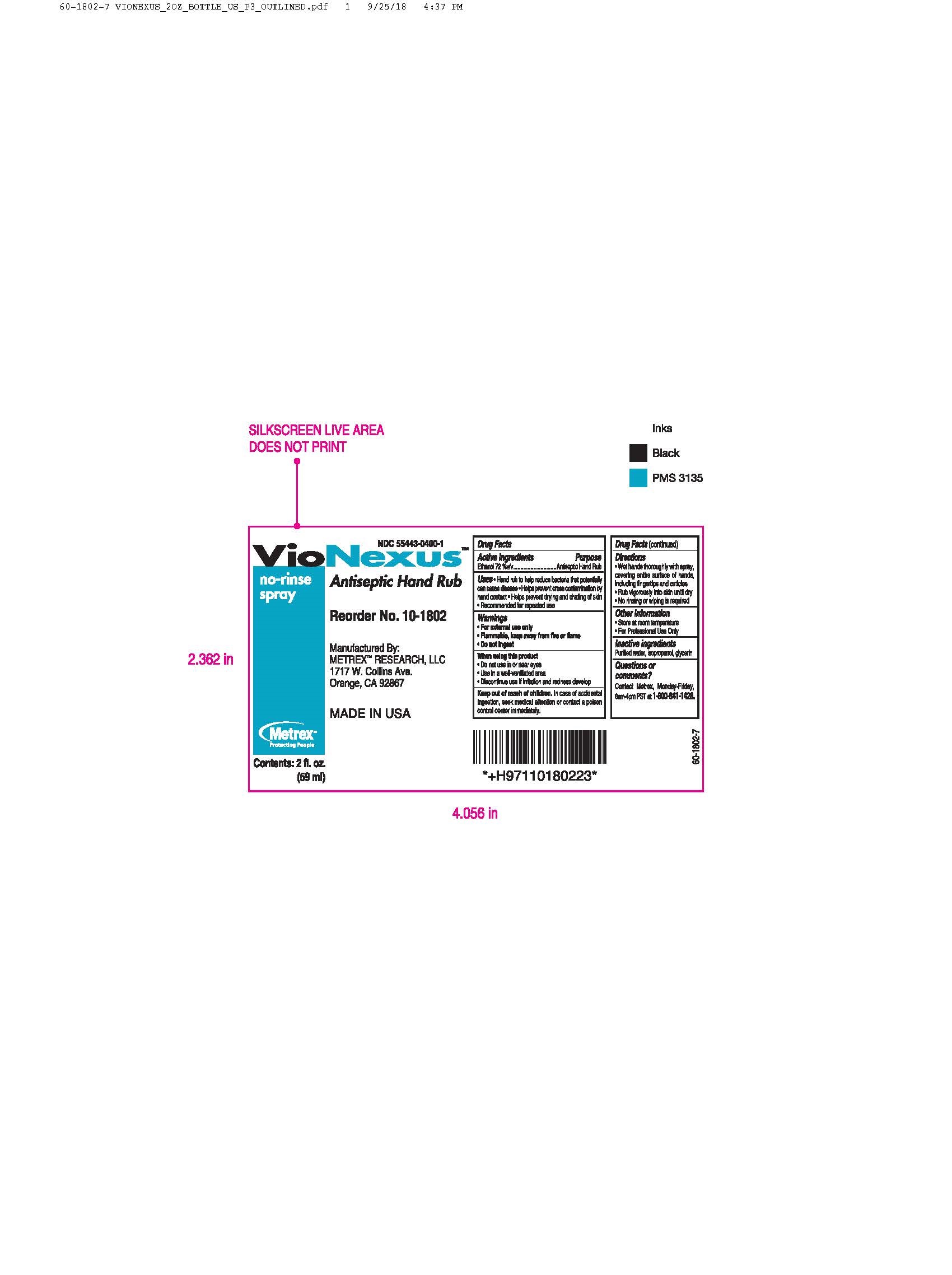
Label: VIONEXUS- alcohol liquid
-
NDC Code(s):
55443-0400-1,
55443-0400-2,
55443-0400-3,
55443-0400-4, view more55443-0400-5, 55443-0400-6, 55443-0400-7
- Packager: METREX RESEARCH, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings
- Uses
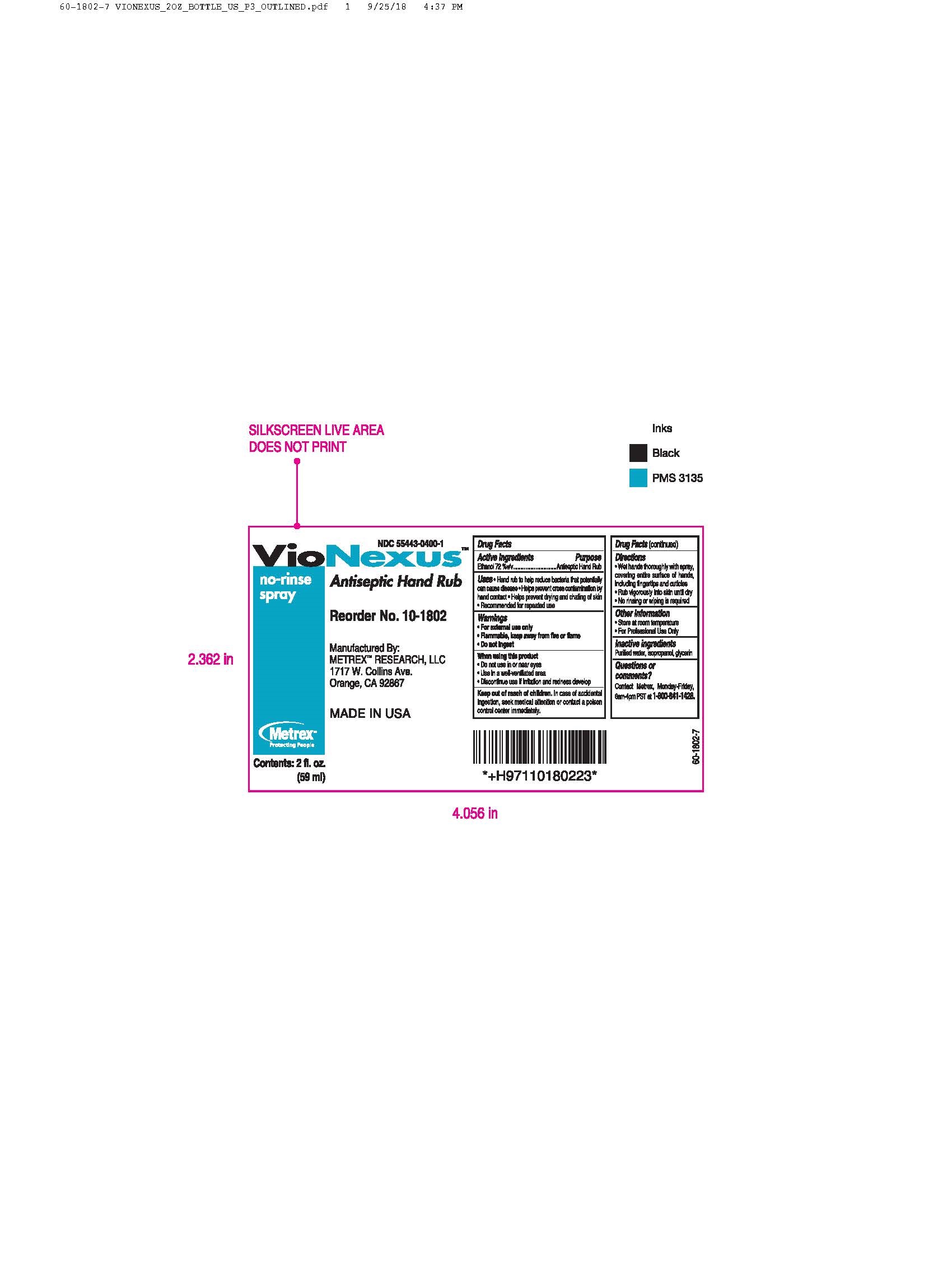
- Active ingredients
- Directions
- Other information
- Inactive ingredients
-
Questions or comments?
For product or technical information, contact Metrex, Monday to Friday, 6am - 4pm PST at 800-841-1428 or visit our website at www.metrex.com.
- Dosage and Administration Route(s)
- Vionexus No Rinse Spray Label
-
INGREDIENTS AND APPEARANCE
VIONEXUS
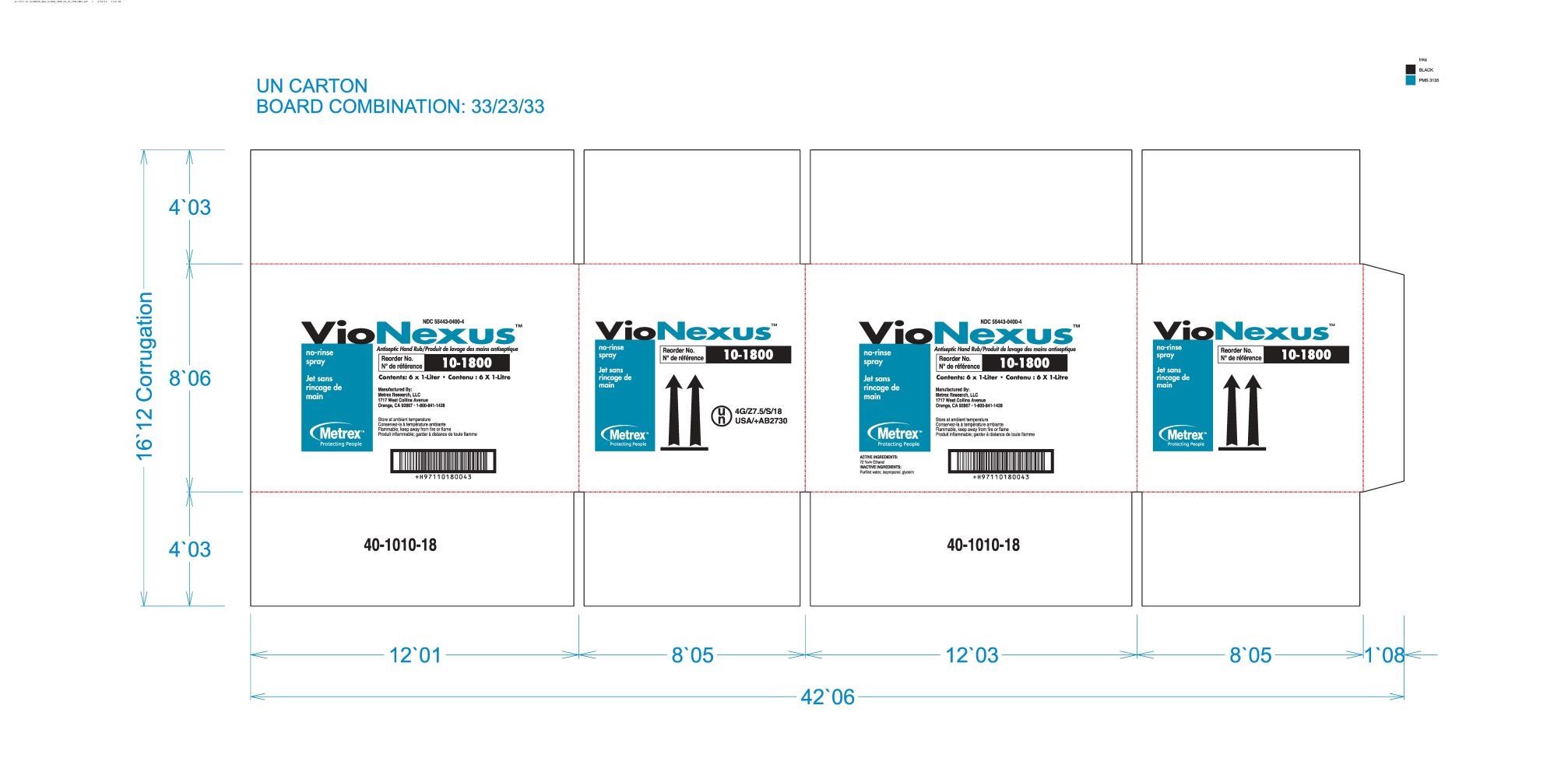
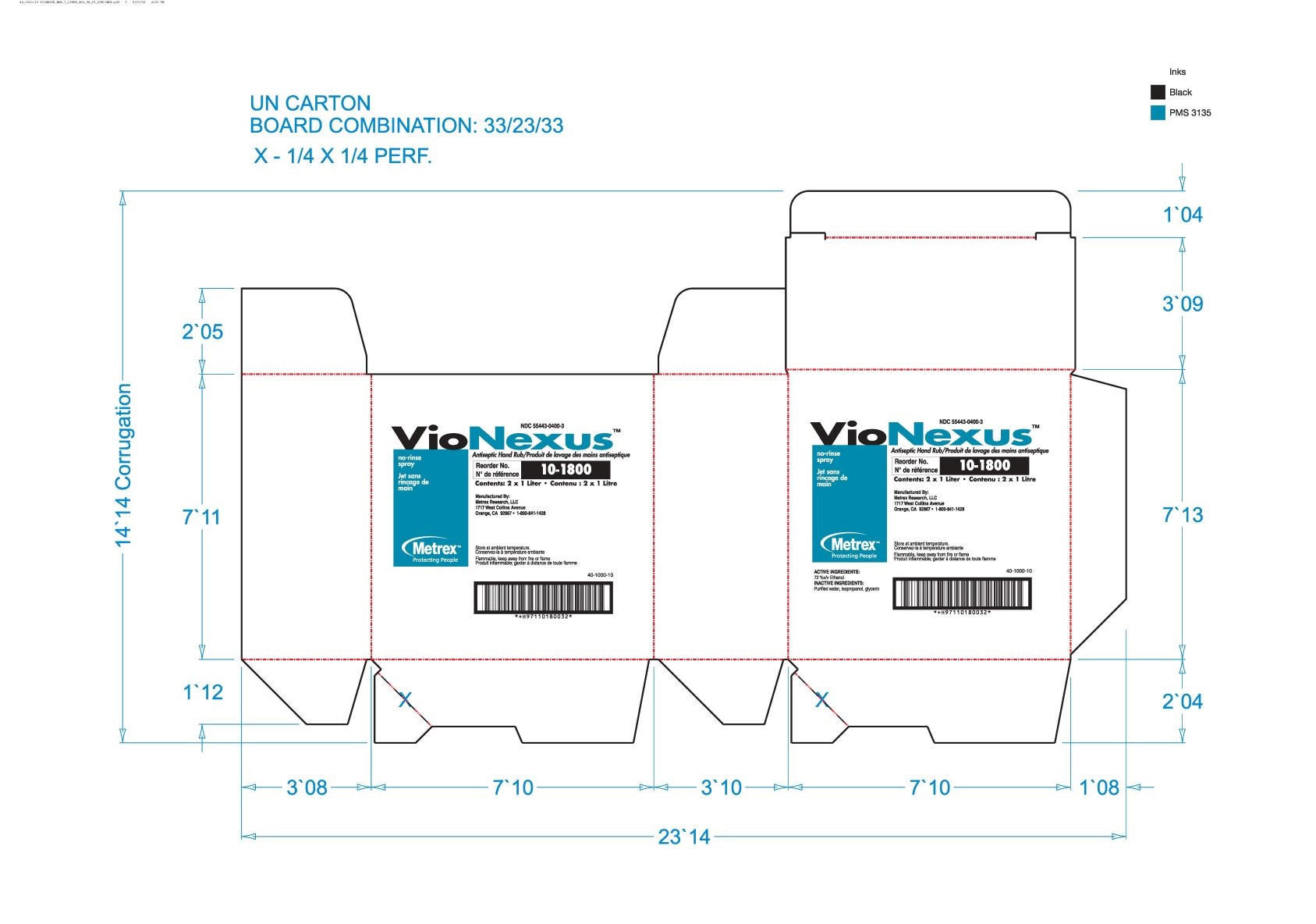
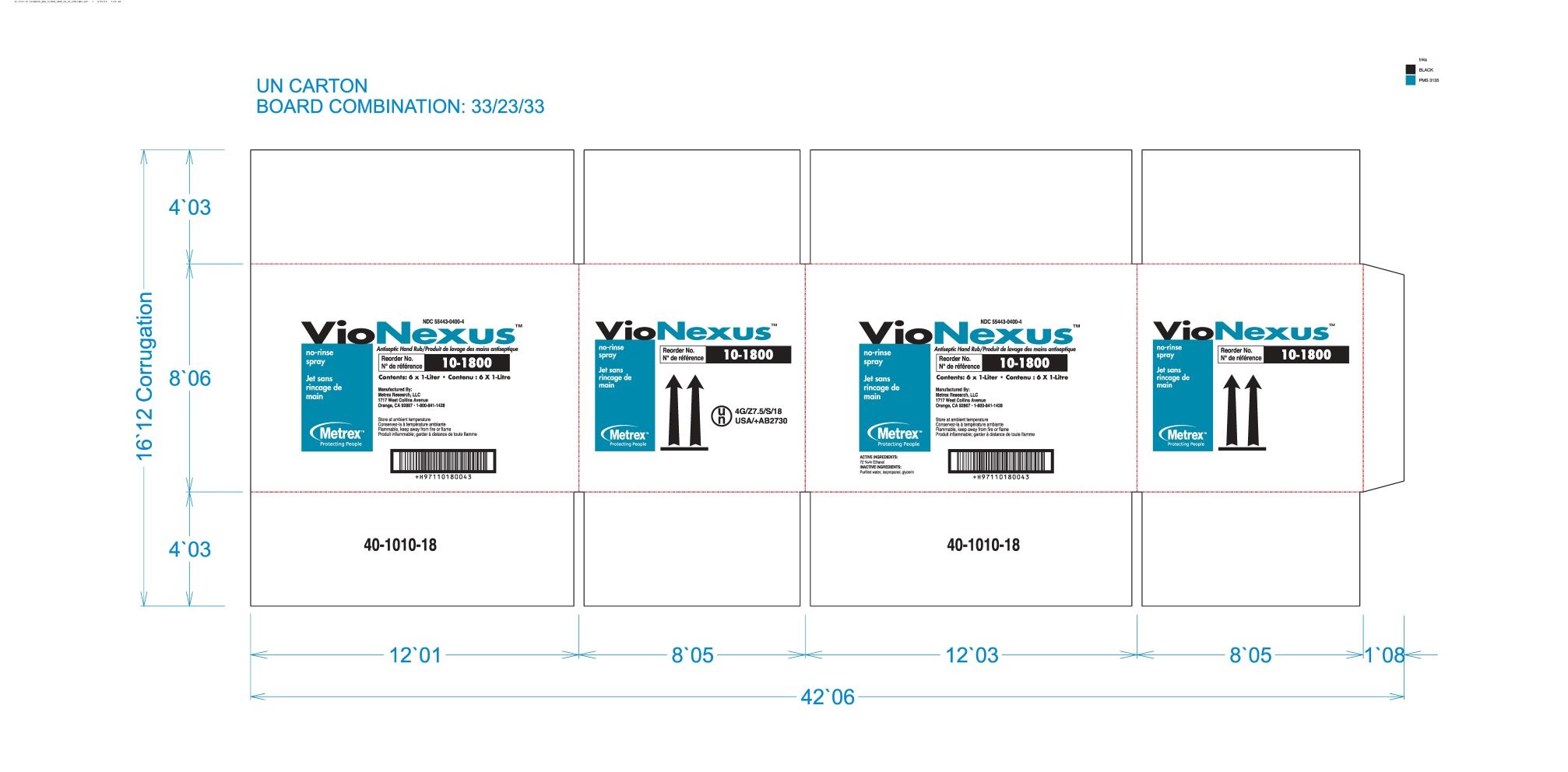
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55443-0400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 72 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL ALCOHOL (UNII: ND2M416302) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55443-0400-5 48 in 1 CASE 11/01/2018 1 NDC:55443-0400-1 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 2 NDC:55443-0400-4 3 in 1 CASE 11/01/2018 2 NDC:55443-0400-3 2 in 1 BOX 2 NDC:55443-0400-2 1000 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 3 NDC:55443-0400-7 12 in 1 CASE 04/01/2020 04/01/2022 3 NDC:55443-0400-6 237 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 11/01/2018 Labeler - METREX RESEARCH, LLC (145963778) Registrant - METREX RESEARCH (145963778) Establishment Name Address ID/FEI Business Operations METREX RESEARCH 145963778 manufacture(55443-0400)