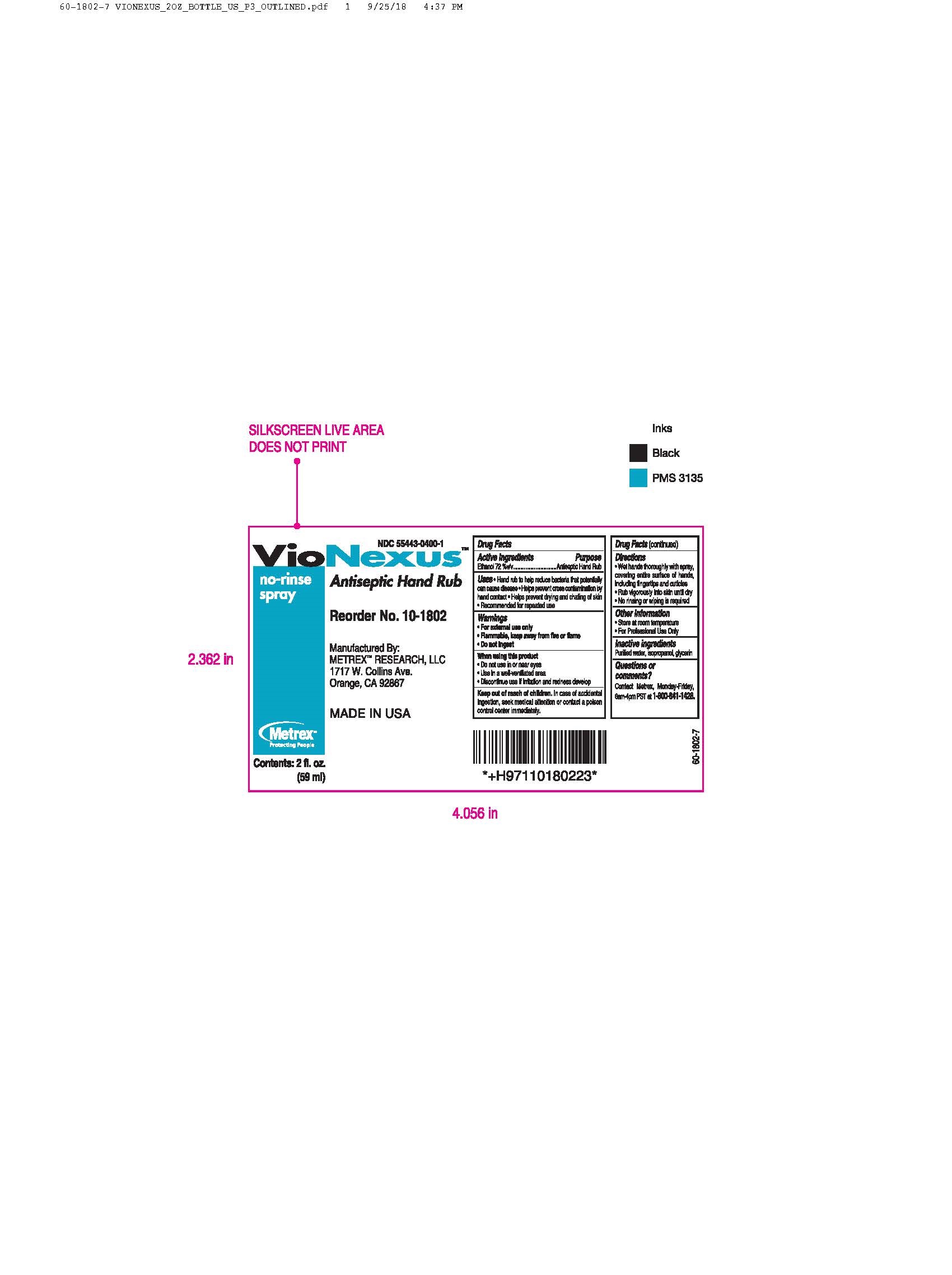
Warnings
- For external use only
- Flammable, keep away from fire or flame
- Do not ingest
Uses
- to help reduce bacteria that potentially can case disease
- helps prevent cross contamination by hand contact
- helps prevent drying and chafing of skin
- recommended for repeated use
Directions
- wet hands thoroughly with spray, covering entire surface of hands, including finger tips and cuticles
- rub vigorously into skin until dry
- no rinsing or wiping is required
Other information
- store at room temperature
- recommended for us in the Vionexus No-Touch Dispenser
- for healthcare and professional use only
Questions or comments?
For product or technical information, contact Metrex, Monday to Friday, 6am - 4pm PST at 800-841-1428 or visit our website at www.metrex.com.