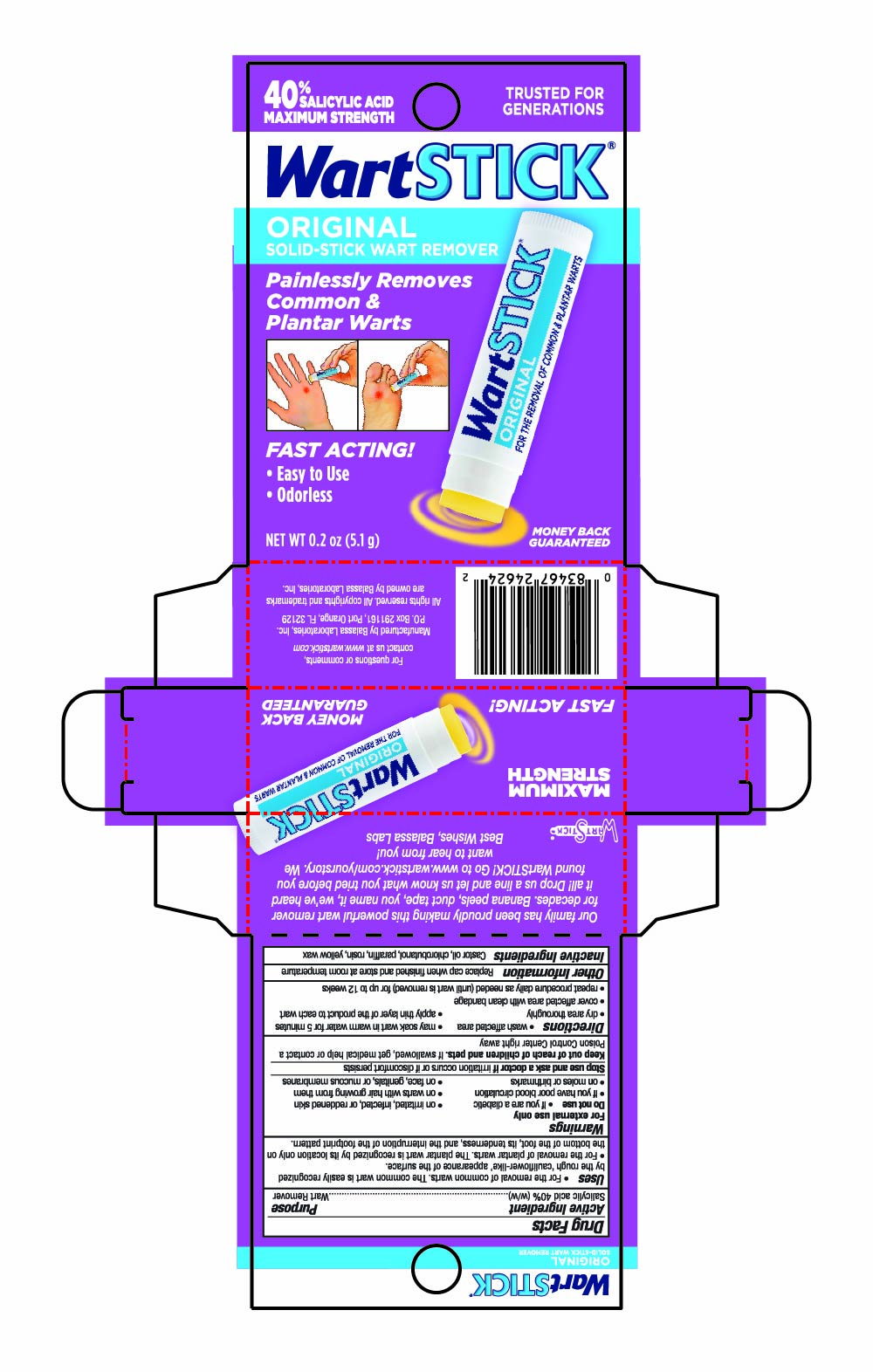
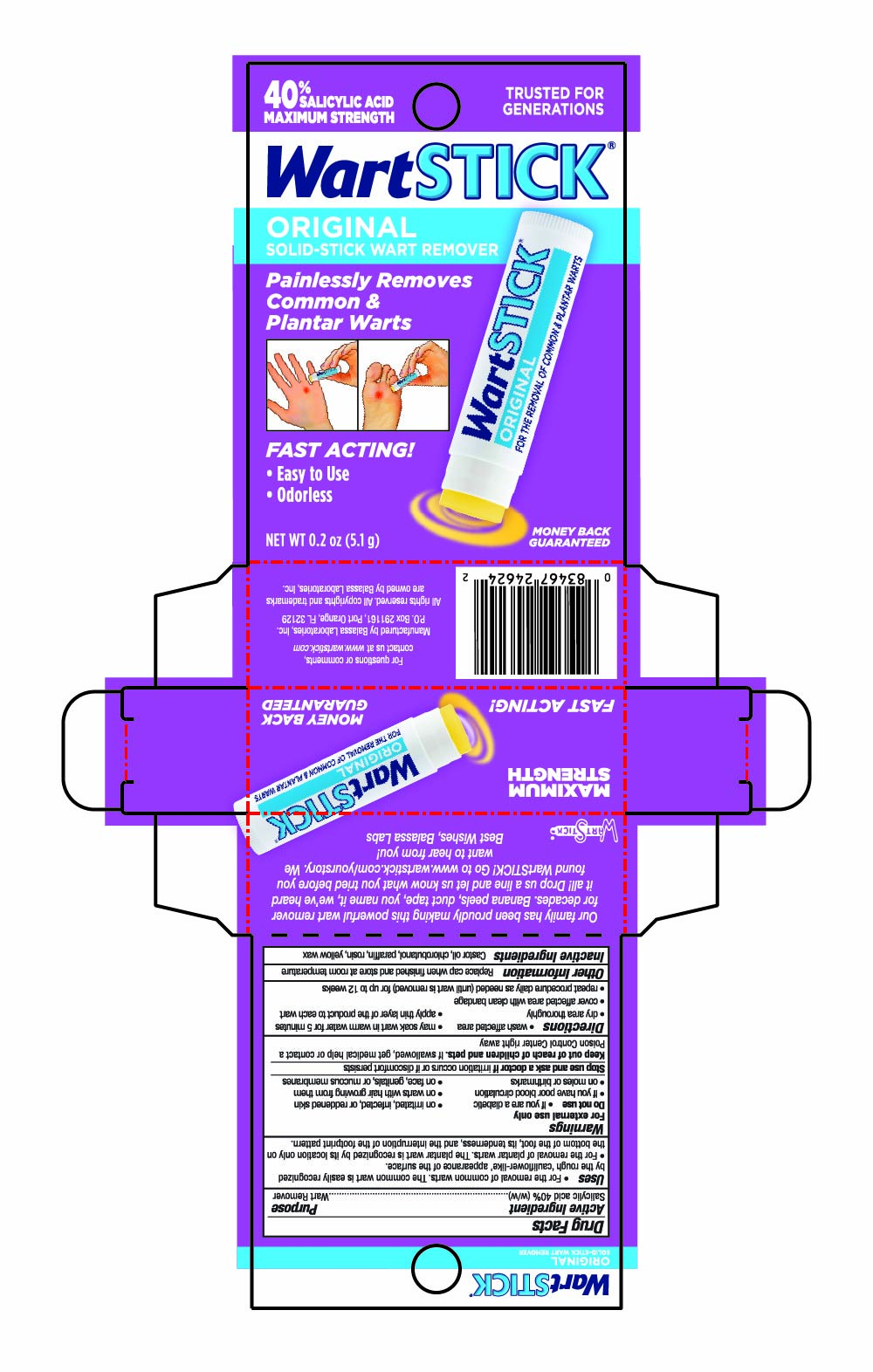
Label: WARTSTICK- salicylic acid stick
- NDC Code(s): 10107-250-01, 10107-250-02, 10107-250-03, 10107-250-08
- Packager: Balassa Laboratories Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Purpose
-
Uses
- For the removal of common warts. The common wart is easily recognized by the rough "cauliflower-like" appearance of the surface.
- For the removal of plantar warts. The plantar wart is recognized by its location only on the bottom of the foot, its tenderness, and the interruption of the footprint pattern.
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL - 5.1 g Tube in Carton - NDC 10107-250-02
- PRINCIPAL DISPLAY PANEL - 5.1 g Tube in Carton - NDC 10107-250-08
- PRINCIPAL DISPLAY PANEL - Two 5.1 g Tubes in Carton
-
INGREDIENTS AND APPEARANCE
WARTSTICK
salicylic acid stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10107-250 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID .4 g in 1 g Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) CHLOROBUTANOL (UNII: HM4YQM8WRC) YELLOW WAX (UNII: 2ZA36H0S2V) PARAFFIN (UNII: I9O0E3H2ZE) ROSIN (UNII: 88S87KL877) Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10107-250-02 1 in 1 CARTON 05/12/2017 1 NDC:10107-250-01 5.1 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10107-250-08 1 in 1 CARTON 08/23/2018 2 NDC:10107-250-01 5.1 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:10107-250-03 2 in 1 CARTON 03/13/2017 3 NDC:10107-250-01 5.1 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358B 06/01/1983 Labeler - Balassa Laboratories Inc. (069162485) Establishment Name Address ID/FEI Business Operations Balassa Laboratories Inc. 069162485 manufacture(10107-250) , label(10107-250) , pack(10107-250)