Uses
- For the removal of common warts. The common wart is easily recognized by the rough "cauliflower-like" appearance of the surface.
- For the removal of plantar warts. The plantar wart is recognized by its location only on the bottom of the foot, its tenderness, and the interruption of the footprint pattern.
Warnings
For external use only.
Directions
- Wash affected area
- May soak in warm water for 5 minutes
- Dry area thoroughly
- Keeping the product off the surrounding skin, apply a thin layer of the product to each wart
- Cover affected area with clean bandage
- Repeat procedure daily as needed (until wart is removed) for up to 12 weeks
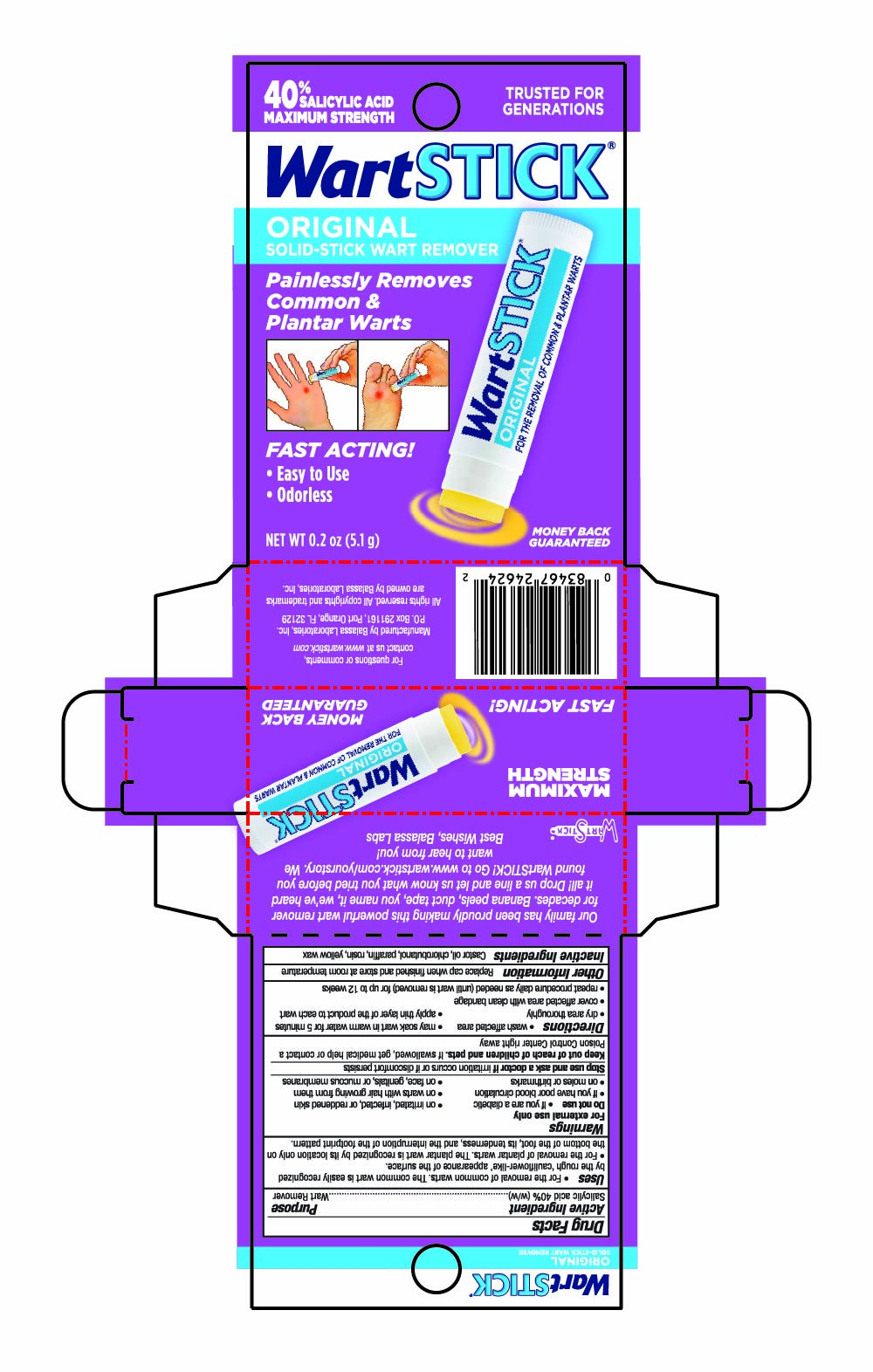
PRINCIPAL DISPLAY PANEL - 5.1 g Tube in Carton - NDC 10107-250-02
40% SALICYLIC ACID
MAXIMUM STRENGTH
TRUSTED FOR GENERATIONS
WartSTICK®
ORIGINAL SOLID-STICK WART REMOVER
Painlessly Removes Common & Plantar Warts
Fast Acting!
- Easy to Use
- Odorless
NET WT 0.2 OZ (5.1g)
MONEY BACK GUARANTEED
PRINCIPAL DISPLAY PANEL - 5.1 g Tube in Carton - NDC 10107-250-08
40% SALICYLIC ACID MAXIMUM STRENGTH
WartSTICK® Solid-Stick Wart Remover
Painlessly Removes Common and Plantar Warts
Fast Acting
- Easy to Use
- Odorless
NET WT 0.2 OZ (5.1g)

PRINCIPAL DISPLAY PANEL - Two 5.1 g Tubes in Carton
40% SALICYLIC ACID
MAXIMUM STRENGTH
TRUSTED FOR GENERATIONS
WartSTICK®
ORIGINAL Solid-Stick Wart Remover
Painlessly Removes Common & Plantar Warts
Fast Acting!
- Easy to Use
- Odorless
Contains: 2 WartSticks
NET WT 0.4 OZ (11.3g)
MONEY BACK GUARANTEED
