Label: SNUGZ HAND SANITIZER GEL- hand sanitizer gel gel
-
NDC Code(s):
76309-301-01,
76309-301-02,
76309-301-04,
76309-301-05, view more76309-301-08, 76309-301-19, 76309-301-51, 76309-301-61, 76309-301-62, 76309-301-81, 76309-301-88, 76309-301-91, 76309-301-99
- Packager: SnugZ/USA, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
-
WARNINGS
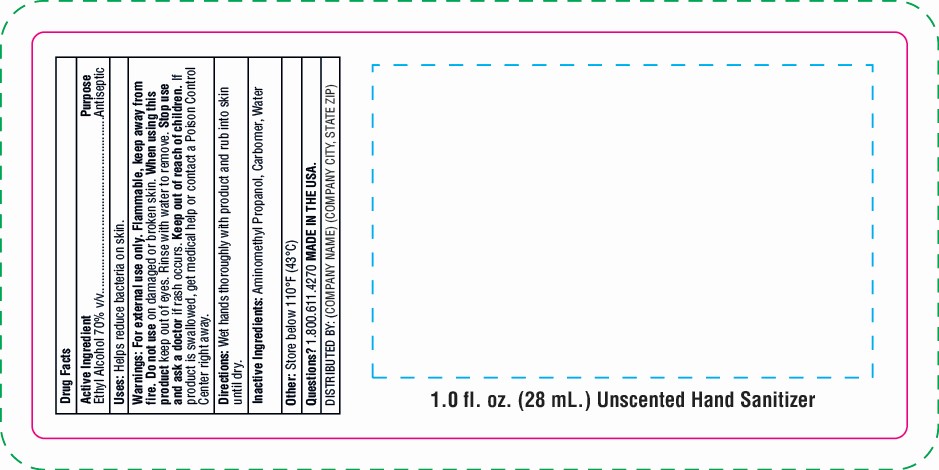
Warnings: For external use only. Flammable, keep away from fire. Do not use on damaged or broken skin. Keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs. Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- Hand Sanitizer Gel
-
INGREDIENTS AND APPEARANCE
SNUGZ HAND SANITIZER GEL
hand sanitizer gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76309-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER 940 (UNII: 4Q93RCW27E) AMINOMETHYL PROPANEDIOL (UNII: CZ7BU4QZJZ) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76309-301-01 28 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 2 NDC:76309-301-02 56 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 3 NDC:76309-301-04 112 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 4 NDC:76309-301-08 224 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/01/2018 5 NDC:76309-301-19 56 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 6 NDC:76309-301-51 30 mL in 1 TUBE; Type 0: Not a Combination Product 01/01/2018 7 NDC:76309-301-05 14 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 8 NDC:76309-301-81 28 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 9 NDC:76309-301-91 28 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 10 NDC:76309-301-61 39.75 mL in 1 POUCH; Type 0: Not a Combination Product 01/01/2018 11 NDC:76309-301-62 85.17 mL in 1 POUCH; Type 0: Not a Combination Product 01/01/2018 12/31/2020 12 NDC:76309-301-99 3785.41 mL in 1 JUG; Type 0: Not a Combination Product 04/20/2020 13 NDC:76309-301-88 112 mL in 1 POUCH; Type 0: Not a Combination Product 04/20/2020 12/31/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/01/2018 Labeler - SnugZ/USA, LLC (615959228) Registrant - SnugZ/USA, LLC (615959228) Establishment Name Address ID/FEI Business Operations SnugZ/USA, LLC 615959228 manufacture(76309-301)