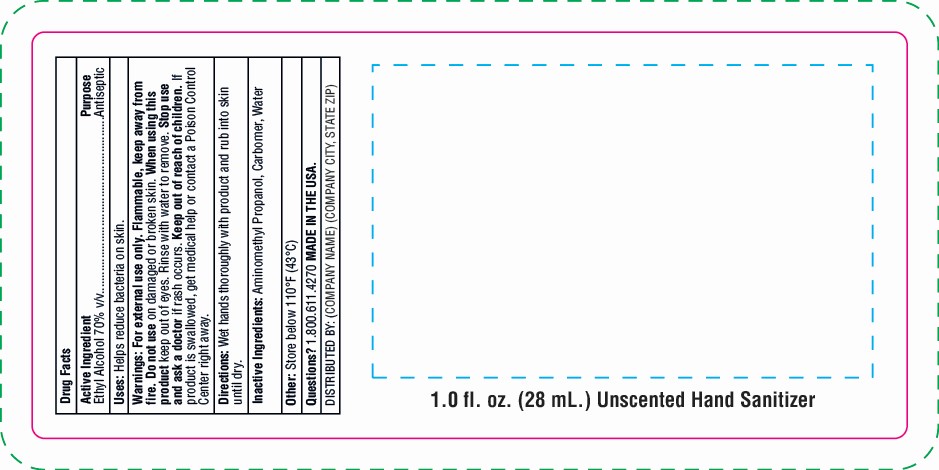
Warnings: For external use only. Flammable, keep away from fire. Do not use on damaged or broken skin. Keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs. Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.