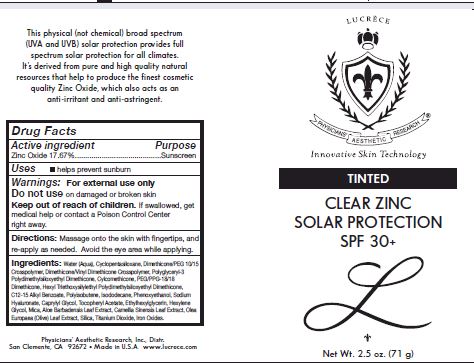
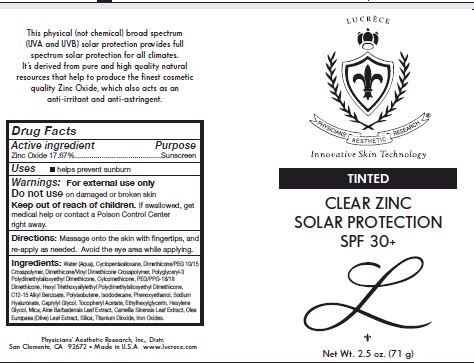
Label: TINTED CLEAR ZINC SOLAR PROTECTION SPF 30- zinc oxide cream
- NDC Code(s): 62742-4112-1
- Packager: Allure Labs, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 4, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Other Ingredients: Water (Aqua), Cyclopentasiloxane, Dimethcone/PEG-10/15 Crosspolymer, Dimethicone/Vinyl Dimethicone Crossploymer, Polyglyceryl-3 polydimethylsiloxyethyl Dimethicone, Cyclomethicone, PEG/PPG-18/18 Dimethicone, Hexyl triethoxysilylethyl Polydimethylsiloxyethyl Dimethicone, C12-15 Alkul benzoate, Poluisobutene, Isododecane, Phenoxyethanol, Sodium Hyaluronate, Caprylyl Glycol, Tocopheryl Acetate, Ethylhexylglycerin, Hexylene Glycol, Mica, Aloe barbadensis Leaf extract, Camellia Sinensis Leaf extract, Olea Europaea (Olive) Leaf Extarct, Silica, Titanium Dioxide, Iron Oxides
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TINTED CLEAR ZINC SOLAR PROTECTION SPF 30
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62742-4112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 176.7 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S) (UNII: RLA2U05Z4Q) CYCLOMETHICONE (UNII: NMQ347994Z) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) MICA (UNII: V8A1AW0880) ALOE (UNII: V5VD430YW9) CAMELLIA SINENSIS FLOWER (UNII: 9I2BJY2J17) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62742-4112-1 71 g in 1 TUBE; Type 0: Not a Combination Product 12/04/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 12/04/2017 Labeler - Allure Labs, Inc (926831603) Registrant - Allure Labs, Inc (926831603) Establishment Name Address ID/FEI Business Operations Allure Labs, Inc 926831603 manufacture(62742-4112)