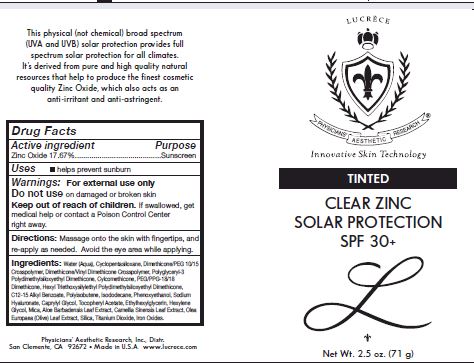
Directions: Masasge onto skin with fingertips, and reapply as needed. Avoid eye area while applying.
For external use only.
Other Ingredients: Water (Aqua), Cyclopentasiloxane, Dimethcone/PEG-10/15 Crosspolymer, Dimethicone/Vinyl Dimethicone Crossploymer, Polyglyceryl-3 polydimethylsiloxyethyl Dimethicone, Cyclomethicone, PEG/PPG-18/18 Dimethicone, Hexyl triethoxysilylethyl Polydimethylsiloxyethyl Dimethicone, C12-15 Alkul benzoate, Poluisobutene, Isododecane, Phenoxyethanol, Sodium Hyaluronate, Caprylyl Glycol, Tocopheryl Acetate, Ethylhexylglycerin, Hexylene Glycol, Mica, Aloe barbadensis Leaf extract, Camellia Sinensis Leaf extract, Olea Europaea (Olive) Leaf Extarct, Silica, Titanium Dioxide, Iron Oxides