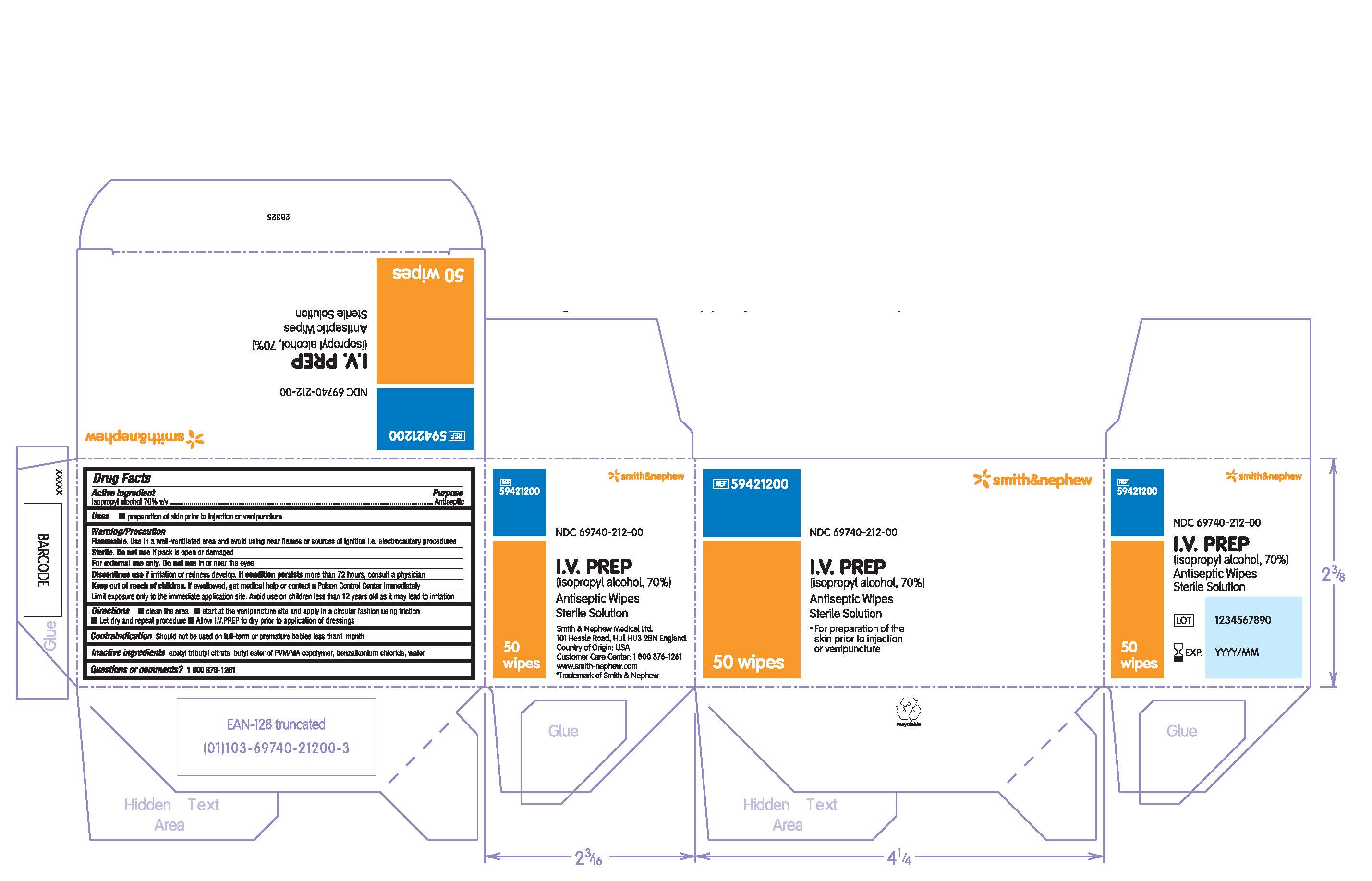
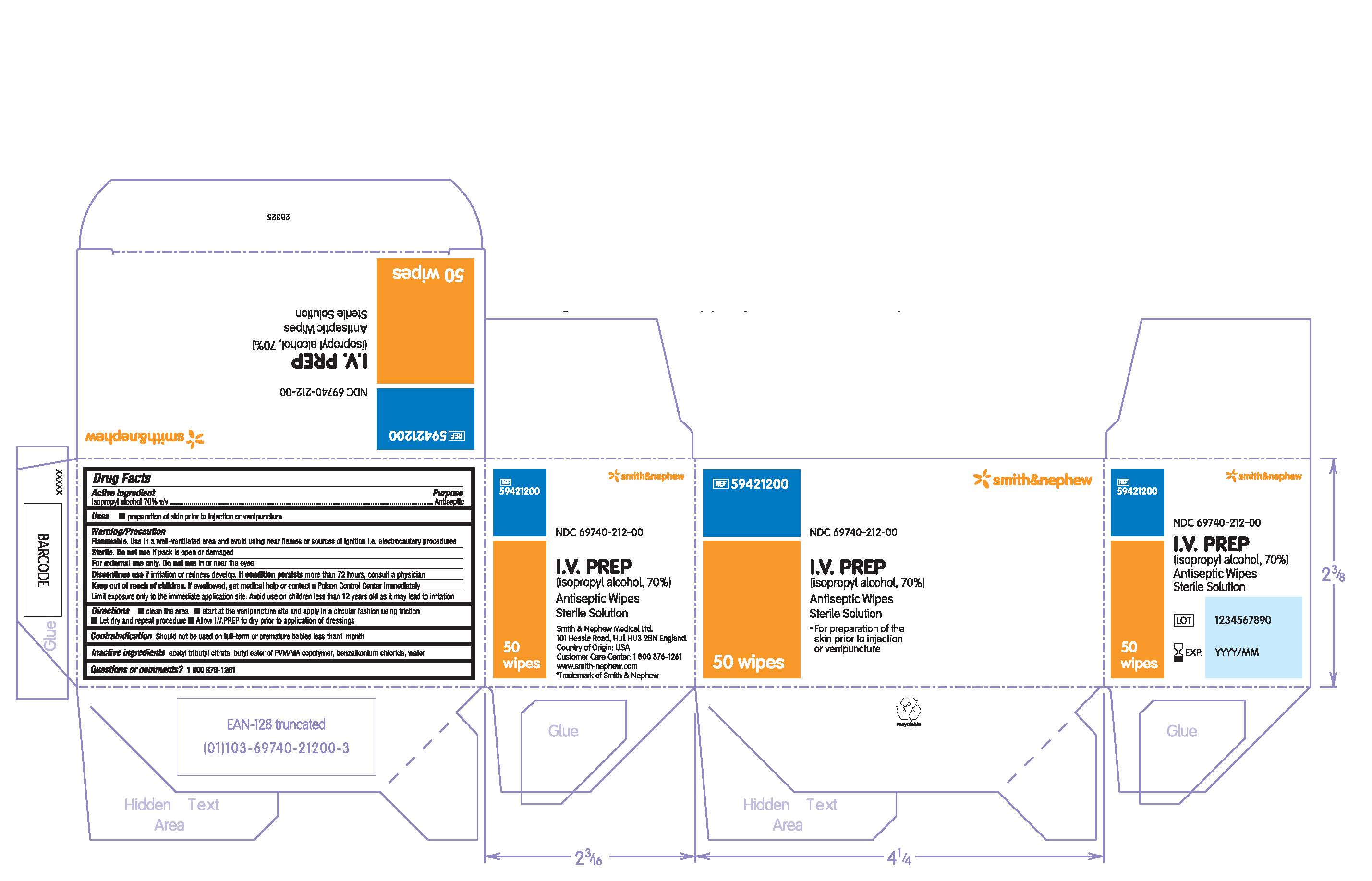
Label: I.V. PREP ANTISEPTIC WIPE- isopropyl alcohol solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 69740-212-00 - Packager: Smith & Nephew, Medical Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 23, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- ACTIVE INGREDIENTS (IN EACH PACKET)
- PURPOSE
- USES
-
WARNINGS
- FLAMMABLE Use in a well-ventilated area and avoid using near flames or sources of ignition i.e. electrocautery procedures
- Sterile. Do not use if pack is open or damaged
- For external use only.Do not use in or near the eyes
- Discontinue use if irritation or redness develop. If condition persists more than 72 hours, consult a physician
- Limit exposure only to the immediate application site. Avoid use on children less than 12 years old as it may lead to irritation
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- CONTRAINDICATION
- INACTIVE INGREDIENTS
- QUESTIONS
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL- BOX OF 50 WIPES (Front)
#59421200
NDC 69740-212-00
I.V. PREP
(isopropyl alcohol, 70%)
Antiseptic Wipes
Sterile Solution
- For preparation of the skin prior to injection or venipuncture
50 Wipes
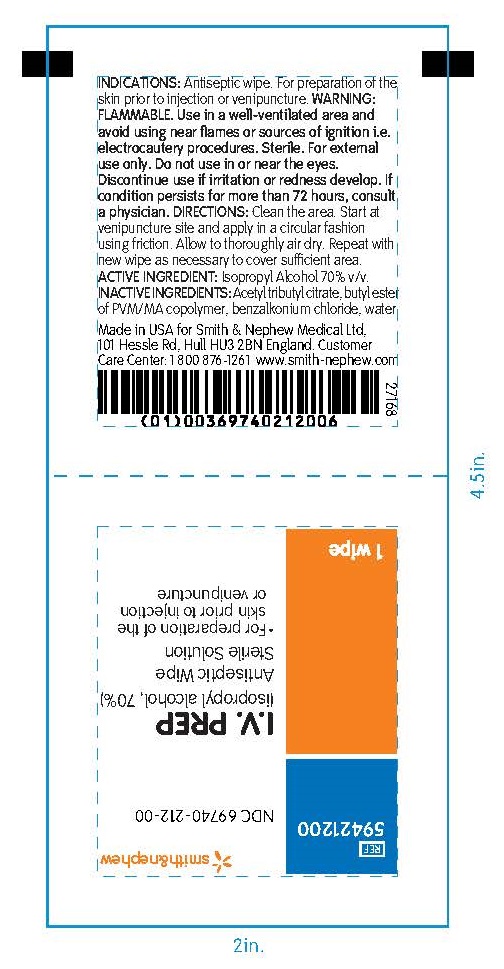
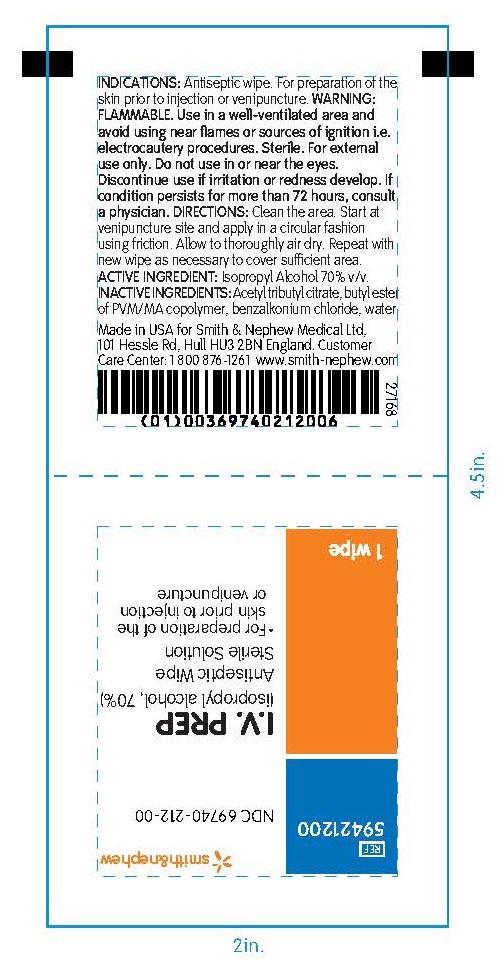
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL- 1 Packet
#59421200
NDC 69740-212-00
I.V. PREP
(isopropyl alcohol, 70%)
Antiseptic Wipe
Sterile Solution
- For preparation of the skin prior to injection or venipuncture
1 Wipe
Made in the USA for Smith & Nephew Medical Ltd
101 Hessle Road, Hull, HU3 2BN, England
Customer Care Center: 1 800 876-1261
www.smith-nephew.com
-
INGREDIENTS AND APPEARANCE
I.V. PREP ANTISEPTIC WIPE
isopropyl alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69740-212 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.57 Inactive Ingredients Ingredient Name Strength ACETYLTRIBUTYL CITRATE (UNII: 0ZBX0N59RZ) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69740-212-00 50 in 1 BOX 10/01/1999 1 1 in 1.0 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 10/01/1999 Labeler - Smith & Nephew, Medical Ltd (216344051) Establishment Name Address ID/FEI Business Operations Span Packaging Solutions 557434805 MANUFACTURE(69740-212)