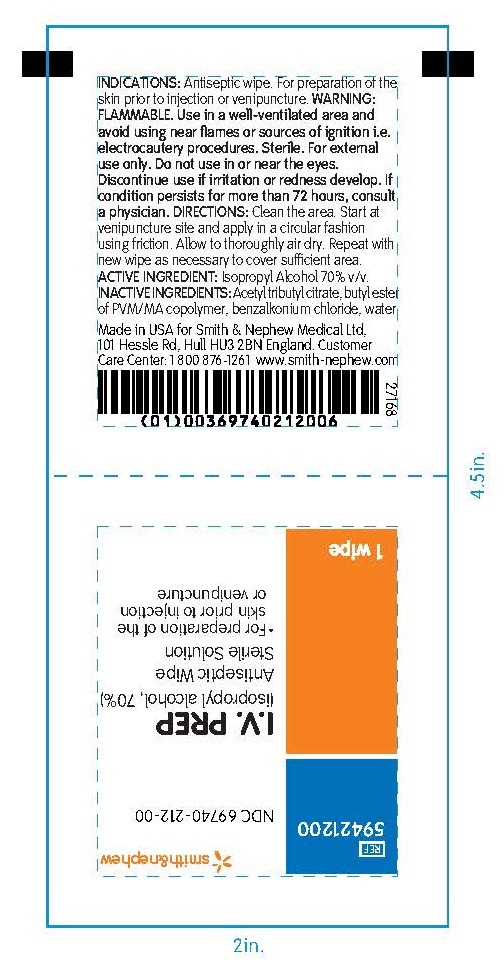
WARNINGS
- FLAMMABLE Use in a well-ventilated area and avoid using near flames or sources of ignition i.e. electrocautery procedures
- Sterile. Do not use if pack is open or damaged
- For external use only.Do not use in or near the eyes
- Discontinue use if irritation or redness develop. If condition persists more than 72 hours, consult a physician
- Limit exposure only to the immediate application site. Avoid use on children less than 12 years old as it may lead to irritation
Keep out of reach of children. If swallowed, get medical help or contact a Poisin Control Center immediately
DIRECTIONS
- clean the area
- start at the venipuncture site and apply in a circular fashion using friction
- Let dry and repeat procedure
- Allow I.V. PREP to dry prior to application of dressings
INACTIVE INGREDIENTS
acetyl tributyl citrate, butyl ester of PVM/MA copolymer, benzalkonium chloride, water
Made in the USA for Smith & Nephew Medical Ltd
101 Hessle Road, Hull, HU3 2BN, England
Customer Care Center: 1 800 876-1261
www.smith-nephew.com
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL- BOX OF 50 WIPES (Front)
#59421200
NDC 69740-212-00
I.V. PREP
(isopropyl alcohol, 70%)
Antiseptic Wipes
Sterile Solution
- For preparation of the skin prior to injection or venipuncture
50 Wipes
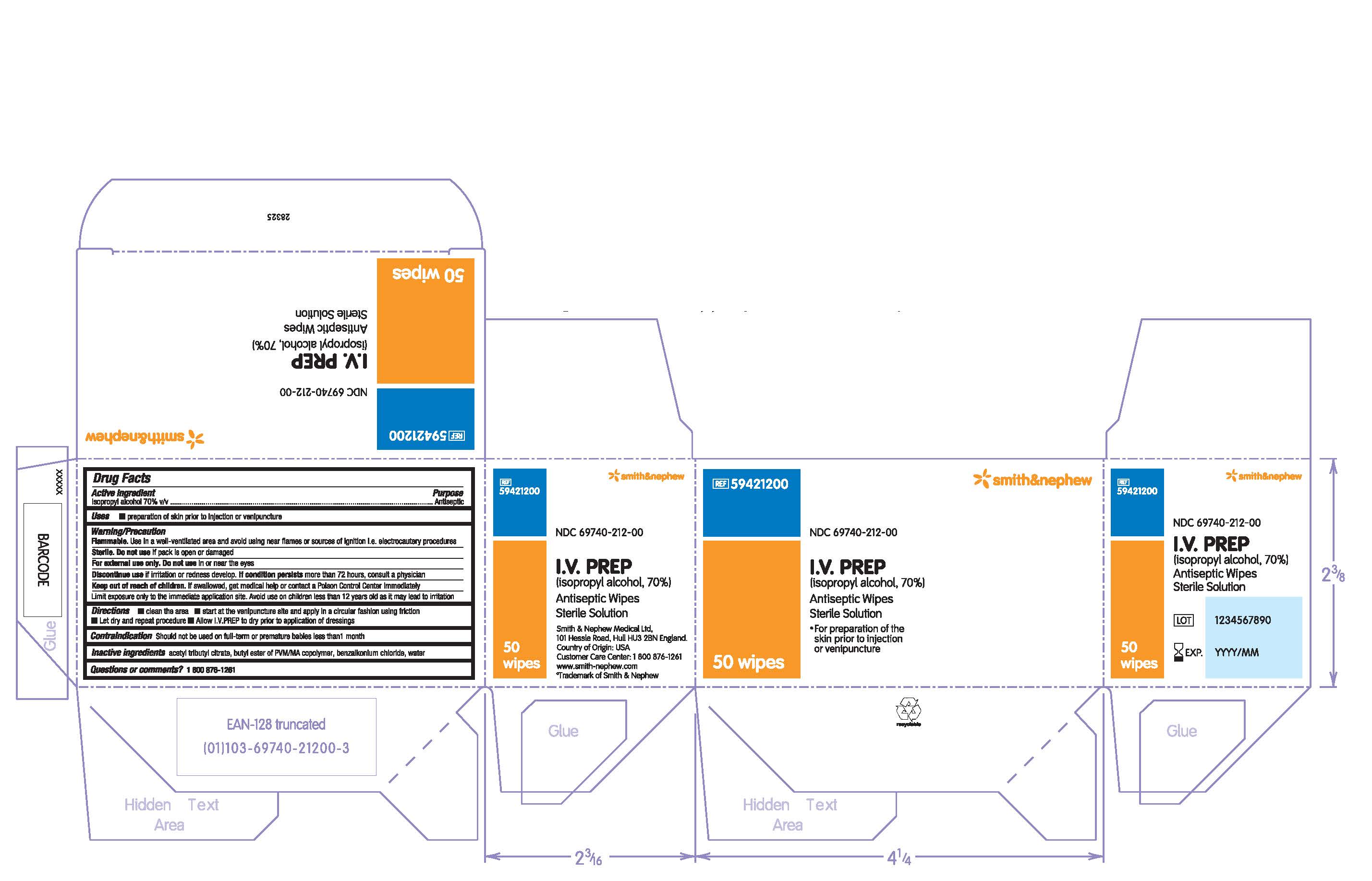
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL- 1 Packet
#59421200
NDC 69740-212-00
I.V. PREP
(isopropyl alcohol, 70%)
Antiseptic Wipe
Sterile Solution
- For preparation of the skin prior to injection or venipuncture
1 Wipe
Made in the USA for Smith & Nephew Medical Ltd
101 Hessle Road, Hull, HU3 2BN, England
Customer Care Center: 1 800 876-1261
www.smith-nephew.com