Label: DAWNMIST ALCOHOL HAND SANITIZER- alcohol gel
- NDC Code(s): 65517-1020-1, 65517-1020-2, 65517-1020-3, 65517-1020-4
- Packager: Dukal LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
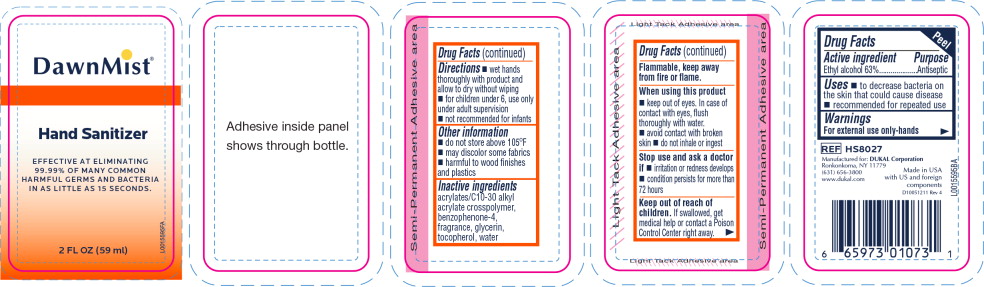
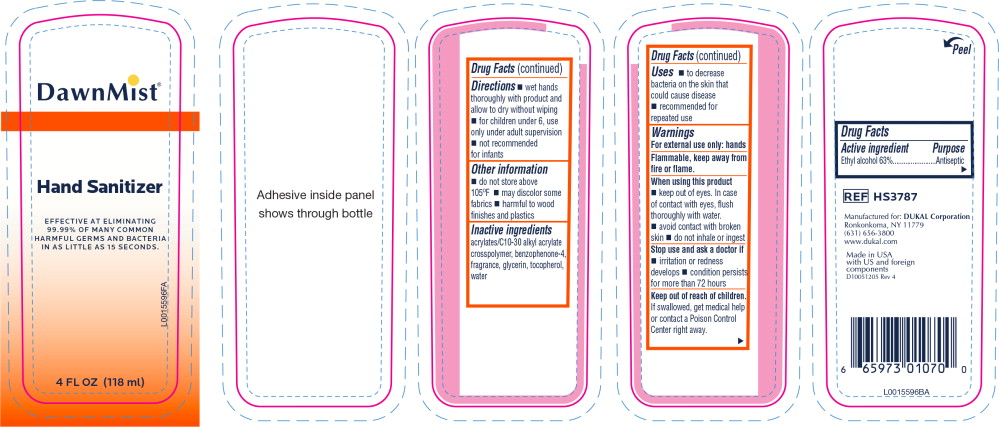
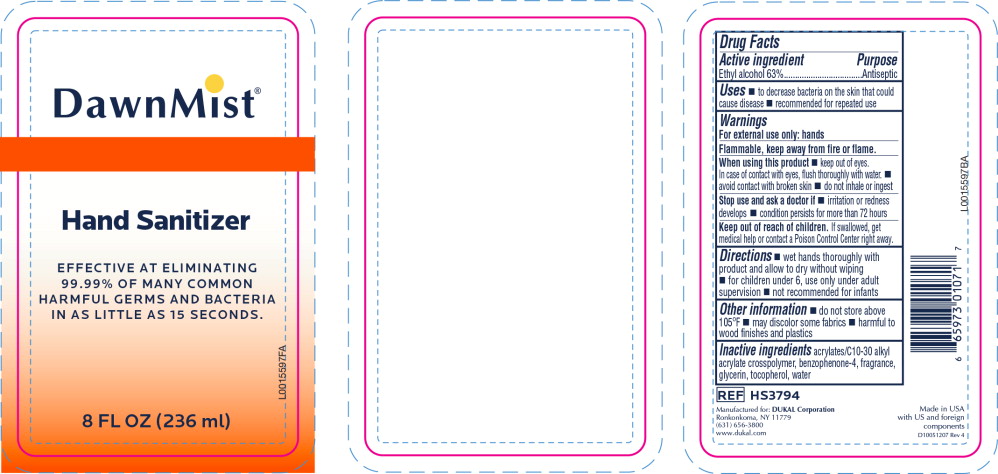
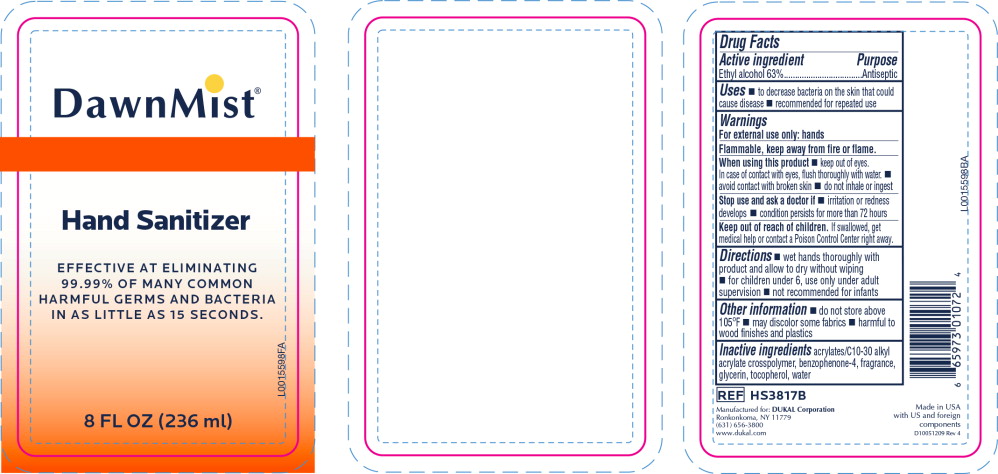
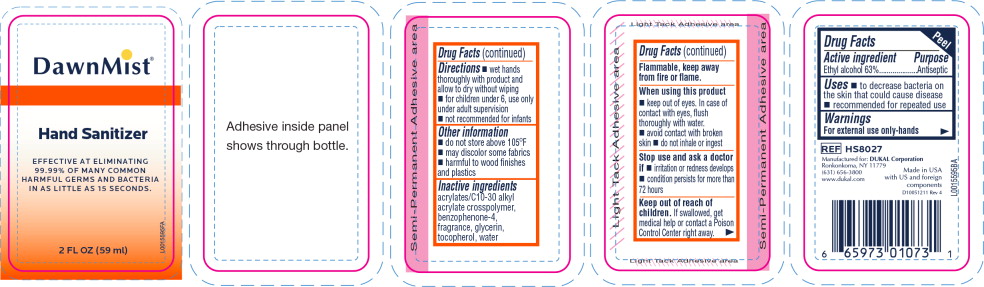
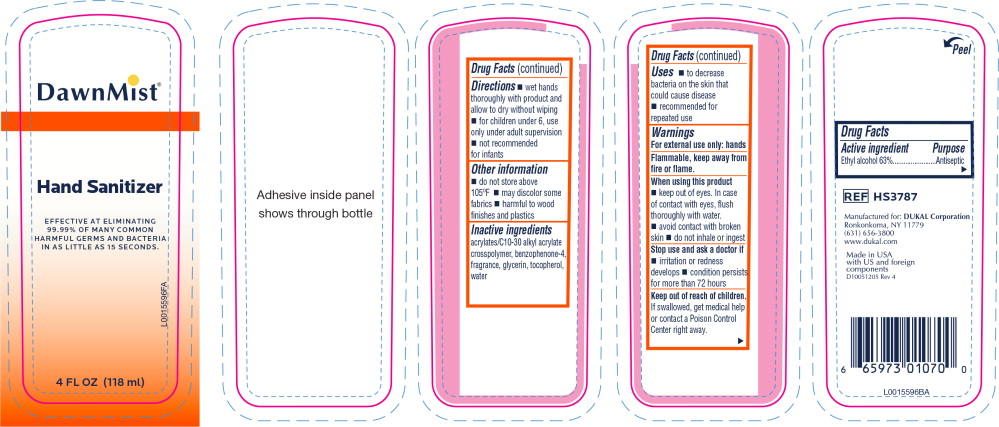
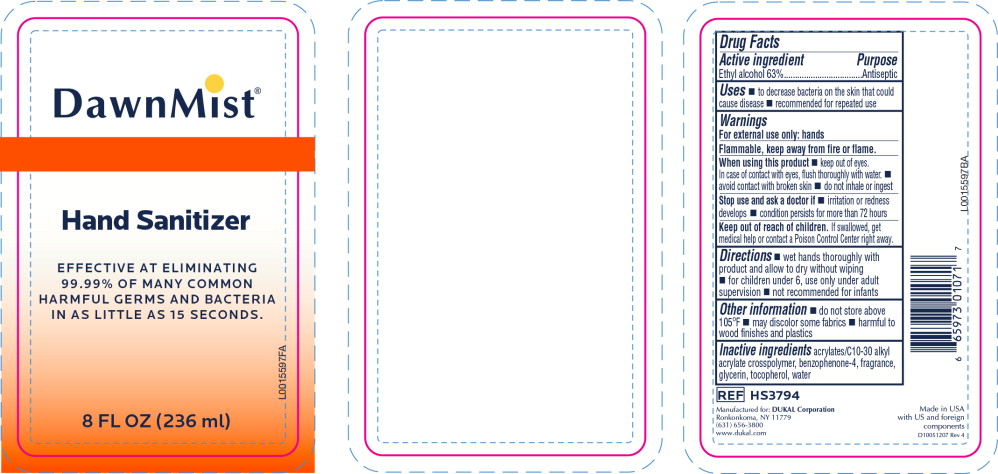
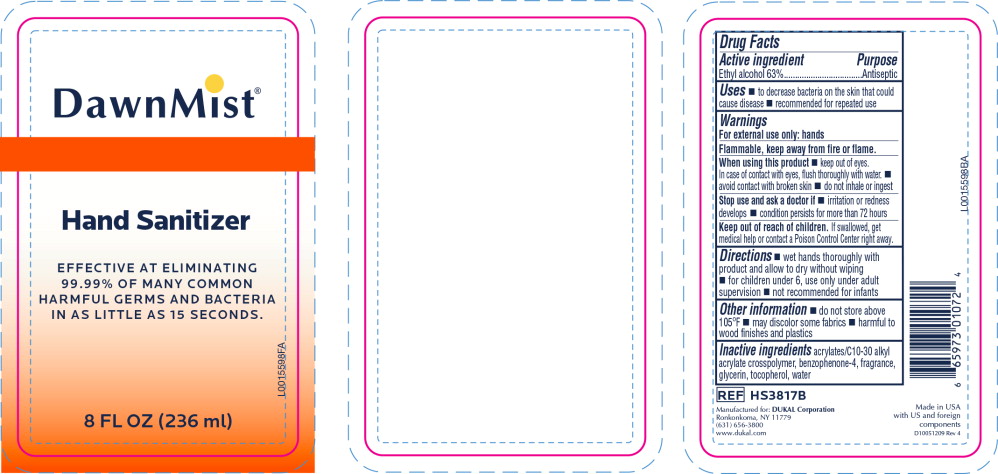
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only: hands
Flammable, keep away from fire or flame.
When using this product
- keep out of eyes. In case of contact with eyes, flush thoroughly with water.
- avoid contact with broken skin
- do not inhale or ingest
- Directions
- Other information
- Inactive ingredients
- Principal Display Panel - DawnMist Alcohol Hand Sanitizer 59 mL Bottle Label
- Principal Display Panel - DawnMist Alcohol Hand Sanitizer 118 mL Bottle Label
- Principal Display Panel - DawnMist Alcohol Hand Sanitizer 236 mL Bottle Label
- Principal Display Panel - DawnMist Alcohol Hand Sanitizer 236 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
DAWNMIST ALCOHOL HAND SANITIZER
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65517-1020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.63 mL in 1 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) SULISOBENZONE (UNII: 1W6L629B4K) GLYCERIN (UNII: PDC6A3C0OX) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-1020-1 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/14/2013 2 NDC:65517-1020-2 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/14/2013 3 NDC:65517-1020-3 236 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/14/2013 4 NDC:65517-1020-4 236 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/14/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/14/2013 Labeler - Dukal LLC (791014871)