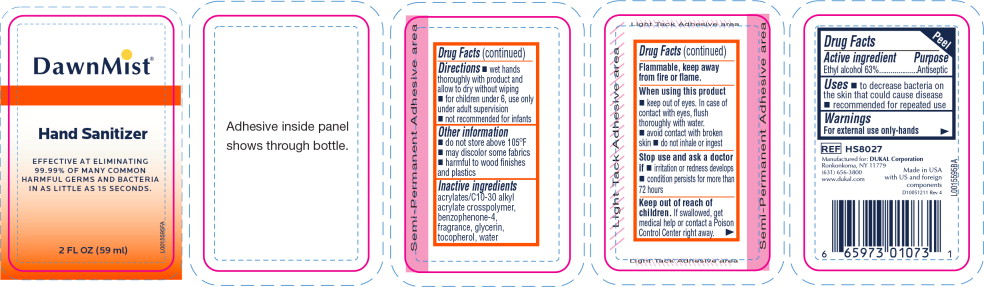
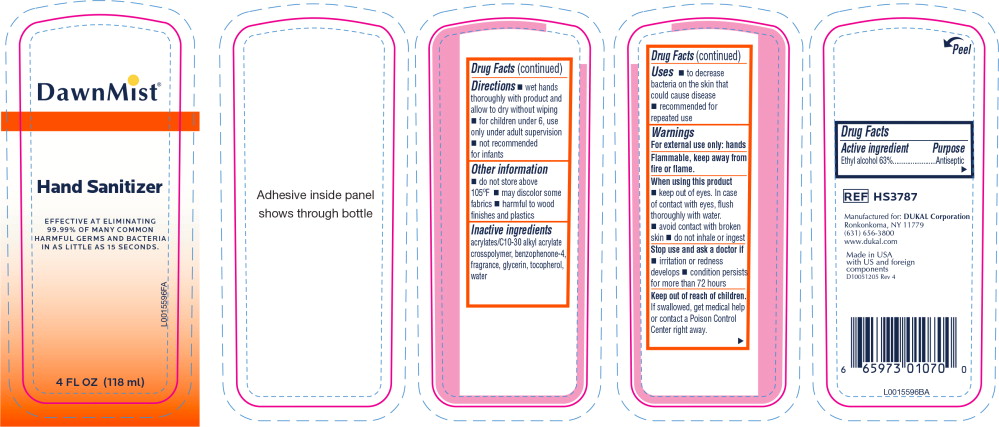
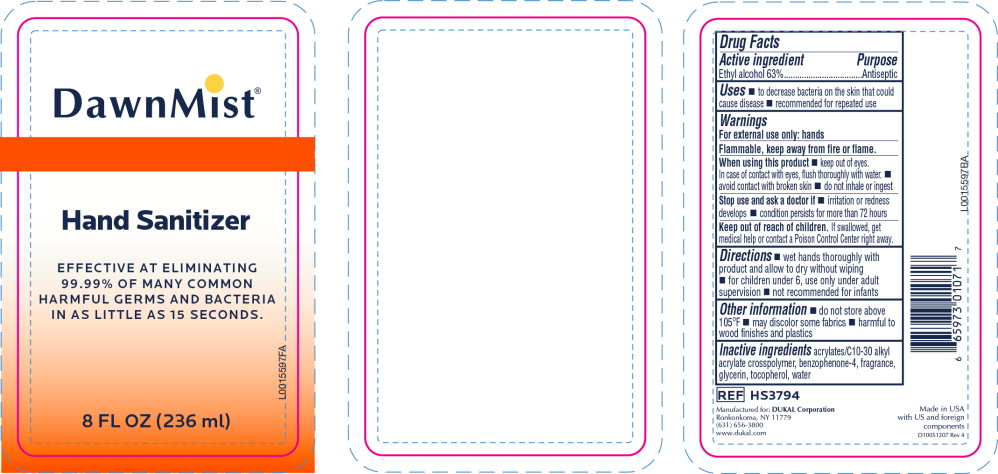
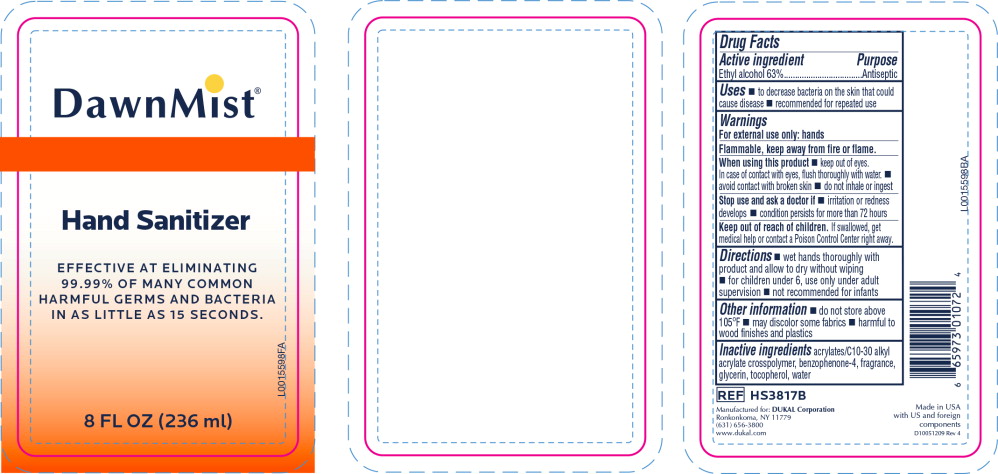
Warnings
For external use only: hands
Flammable, keep away from fire or flame.
When using this product
- keep out of eyes. In case of contact with eyes, flush thoroughly with water.
- avoid contact with broken skin
- do not inhale or ingest
Directions
- wet hands thoroughly with product and allow to dry without wiping
- for children under 6, use only under adult supervision
- not recommended for infants
Other information
- do not store above 105°F
- may discolor some fabrics
- harmful to wood finishes and plastics
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, benzophenone-4, fragrance, glycerin, tocopherol, water
Principal Display Panel - DawnMist Alcohol Hand Sanitizer 59 mL Bottle Label
DawnMist®
Hand Sanitizer
EFFECTIVE AT ELIMINATING
99.99% OF MANY COMMON
HARMFUL GERMS AND BACTERIA
IN AS LITTLE AS 15 SECONDS.
2 FL. OZ. (59 ml)
Principal Display Panel - DawnMist Alcohol Hand Sanitizer 118 mL Bottle Label
DawnMist®
Hand Sanitizer
EFFECTIVE AT ELIMINATING
99.99% OF MANY COMMON
HARMFUL GERMS AND BACTERIA
IN AS LITTLE AS 15 SECONDS.
4 FL. OZ. (118 ml)