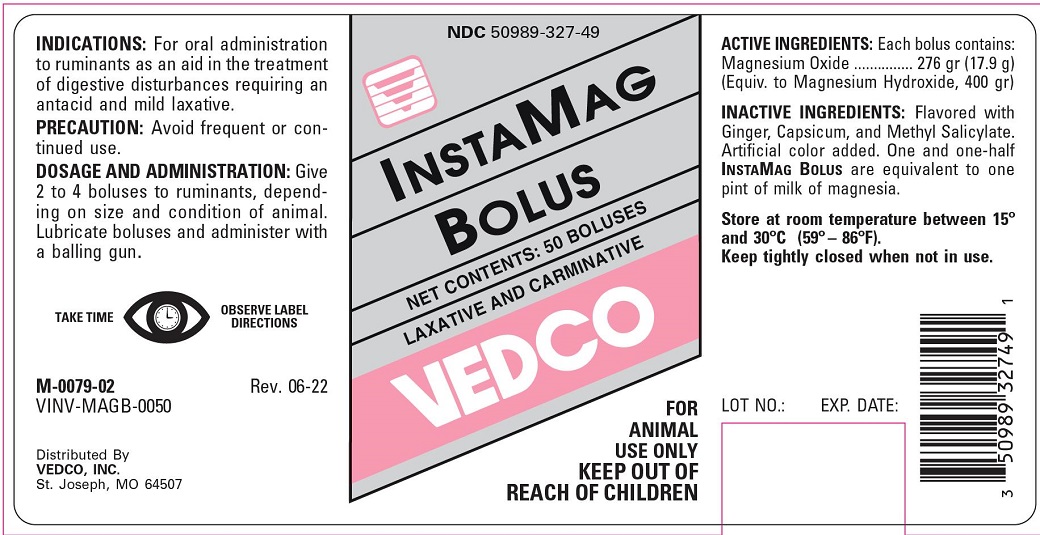
Label: INSTAMAG- magnesium oxide tablet
- NDC Code(s): 50989-327-49
- Packager: Vedco, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- PRECAUTION
- DOSAGE AND ADMINISTRATION
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INSTAMAG
magnesium oxide tabletProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:50989-327 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 276 g Product Characteristics Color pink ( PINK) Score 2 pieces Shape OVAL (OBLONG) Size 8mm Flavor Imprint Code 2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50989-327-49 50 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/05/2005 Labeler - Vedco, Inc. (021634266)