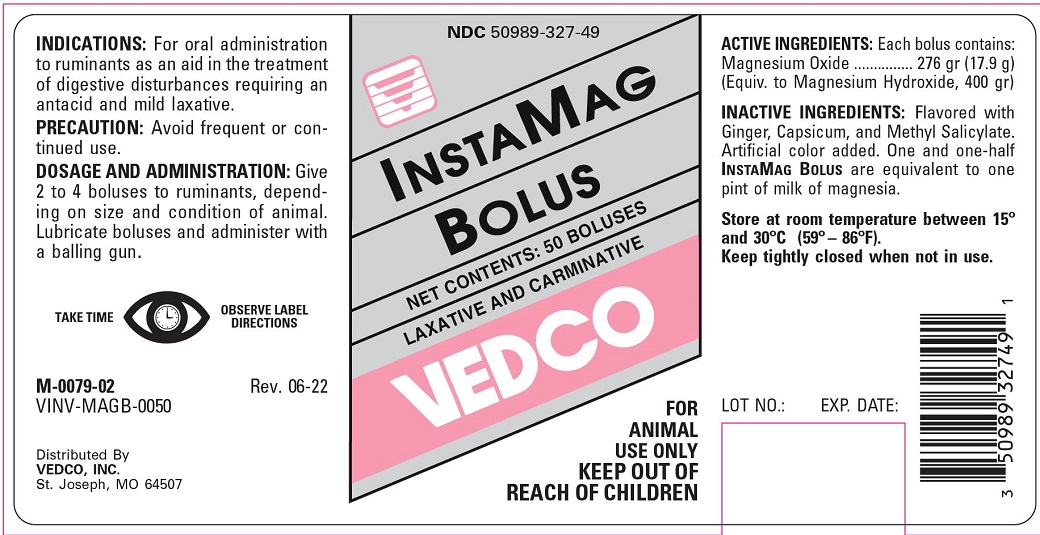
ANTIACID OR MILD LAXATIVE
FOR ANIMAL USE ONLY
KEEP OUT OF REACH OF CHILDREN
INDICATIONS
For oral administration as an aid in the treatment of digestive disturbances requiring an antacid or mild laxative in cattle.
PRECAUTION
Avoid frequent or continued use.
DOSAGE AND ADMINISTRATION
Give 2 to 4 boluses to ruminants, depending on size and condition of animal. Lubricate boluses and adminster with a balling gun.
COMPOSITION
Each bolus contains:
Magnesium Oxide ...... 276 grs. (17.9 g)
(Equivalent to Magnesium Hydroxide, 400 grs.)
Flavored with Ginger, Capsicum, and Methyl Salicylate. Artificial color added. One and one-half InstaMag Bolus are equivalent to one pint of milk of magnesia.
Store at room temperature between 15° and 30°C (59°-86°F).
Keep tightly closed when not in use.
TAKE TIME TO OBSERVE LABEL DIRECTIONS
Vedco, Inc.