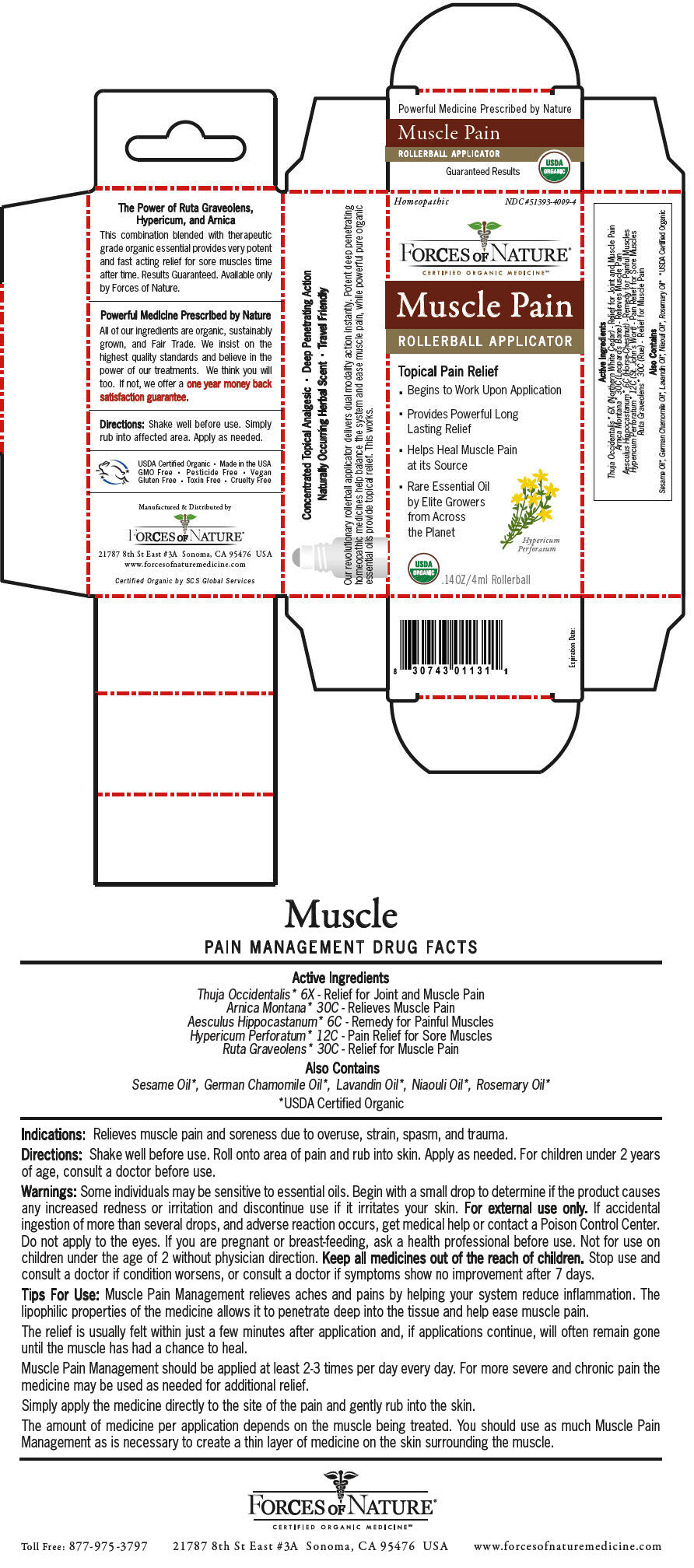
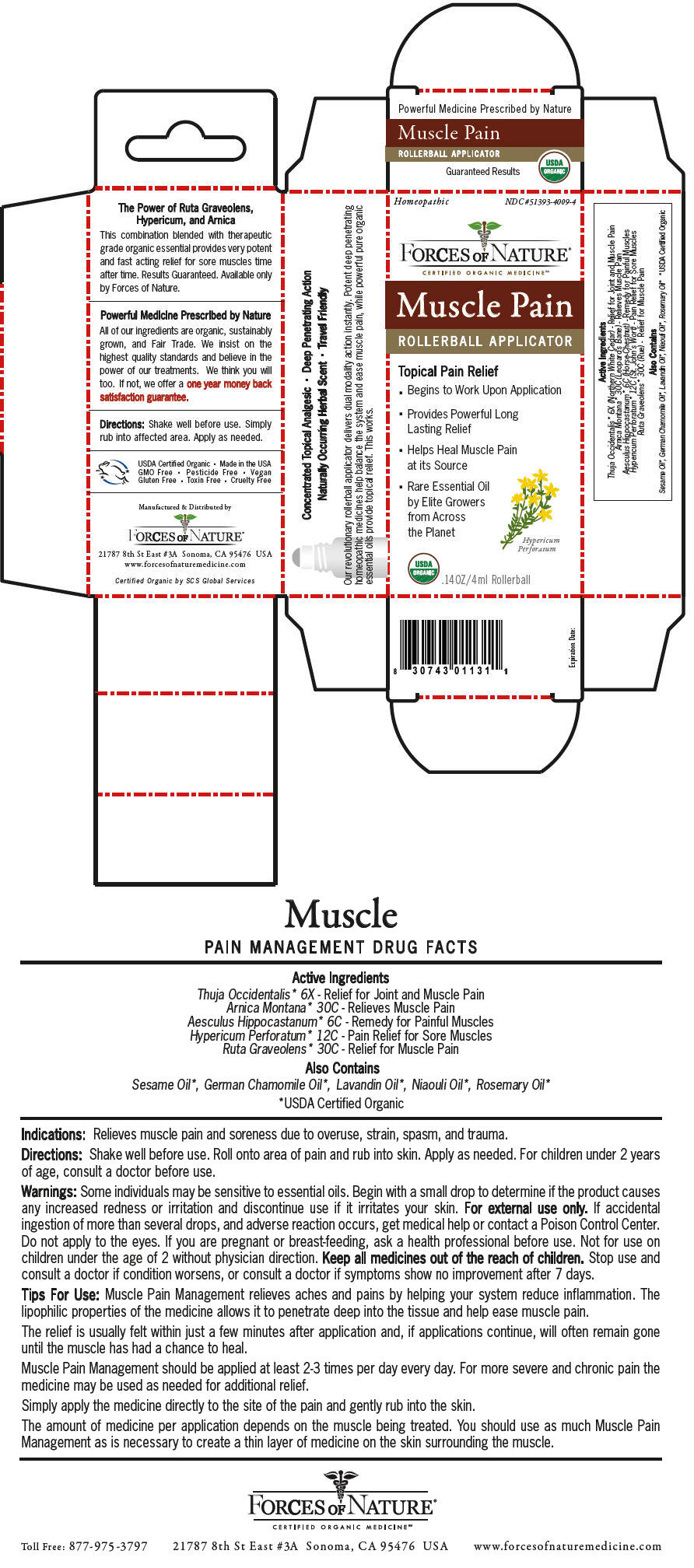
Label: MUSCLE PAIN PAIN MANAGEMENT- arnica montana, horse chestnut, hypericum perforatum, ruta graveolens flowering top, and thuja occidentalis leafy twig solution/ drops
- NDC Code(s): 51393-4009-1, 51393-4009-2, 51393-4009-4
- Packager: Forces of Nature
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 25, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active Ingredients - *
- USDA Certified Organic
Thuja Occidentalis* 6X - Relief for Joint and Muscle Pain Arnica Montana* 30C - Relieves Muscle Pain Aesculus Hippocastanum* 6C - Remedy for Painful Muscles Hypericum Perforatum* 12C - Pain Relief for Sore Muscles Ruta Graveolens* 30C - Relief for Muscle Pain - Indications
- Directions
-
Warnings
Some individuals may be sensitive to essential oils. Begin with a small drop to determine if the product causes any increased redness or irritation and discontinue use if it irritates your skin. For external use only. If accidental ingestion of more than several drops, and adverse reaction occurs, get medical help or contact a Poison Control Center. Do not apply to the eyes. If you are pregnant or breast-feeding, ask a health professional before use. Not for use on children under the age of 2 without physician direction.
-
Tips For Use
Muscle Pain Management relieves aches and pains by helping your system reduce inflammation. The lipophilic properties of the medicine allows it to penetrate deep into the tissue and help ease muscle pain.
The relief is usually felt within just a few minutes after application and, if applications continue, will often remain gone until the muscle has had a chance to heal.
Muscle Pain Management should be applied at least 2-3 times per day every day. For more severe and chronic pain the medicine may be used as needed for additional relief.
Simply apply the medicine directly to the site of the pain and gently rub into the skin.
The amount of medicine per application depends on the muscle being treated. You should use as much Muscle Pain Management as is necessary to create a thin layer of medicine on the skin surrounding the muscle.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 4 ml Bottle Carton
Homeopathic
NDC #51393-4009-4FORCES OF NATURE®
CERTIFIED ORGANIC MEDICINE℠
Muscle Pain
ROLLERBALL APPLICATOR
Topical Pain Relief
- Begins to Work Upon Application
- Provides Powerful Long
Lasting Relief - Helps Heal Muscle Pain
at its Source - Rare Essential Oil
by Elite Growers
from Across
the Planet
Hypericum
PerforatumUSDA
ORGANIC.14OZ/4ml Rollerball
-
INGREDIENTS AND APPEARANCE
MUSCLE PAIN PAIN MANAGEMENT
arnica montana, horse chestnut, hypericum perforatum, ruta graveolens flowering top, and thuja occidentalis leafy twig solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51393-4009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA WHOLE (UNII: O80TY208ZW) (ARNICA MONTANA WHOLE - UNII:O80TY208ZW) ARNICA MONTANA WHOLE 30 [hp_C] in 1000 mL Horse Chestnut (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) Horse Chestnut 6 [hp_C] in 1000 mL HYPERICUM PERFORATUM WHOLE (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM WHOLE - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM WHOLE 12 [hp_C] in 1000 mL Ruta Graveolens Flowering Top (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) Ruta Graveolens Flowering Top 30 [hp_C] in 1000 mL Thuja Occidentalis Leafy Twig (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) Thuja Occidentalis Leafy Twig 6 [hp_X] in 1000 mL Inactive Ingredients Ingredient Name Strength MELALEUCA QUINQUENERVIA LEAF OIL (UNII: 22K2F1YSHD) Chamomile Flower Oil (UNII: 60F80Z61A9) Lavandin Oil (UNII: 9RES347CKG) Rosemary Oil (UNII: 8LGU7VM393) Sesame Oil (UNII: QX10HYY4QV) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51393-4009-4 4 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 02/15/2017 2 NDC:51393-4009-1 11 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/15/2013 3 NDC:51393-4009-2 33 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/15/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 03/15/2013 Labeler - Forces of Nature (050169130)