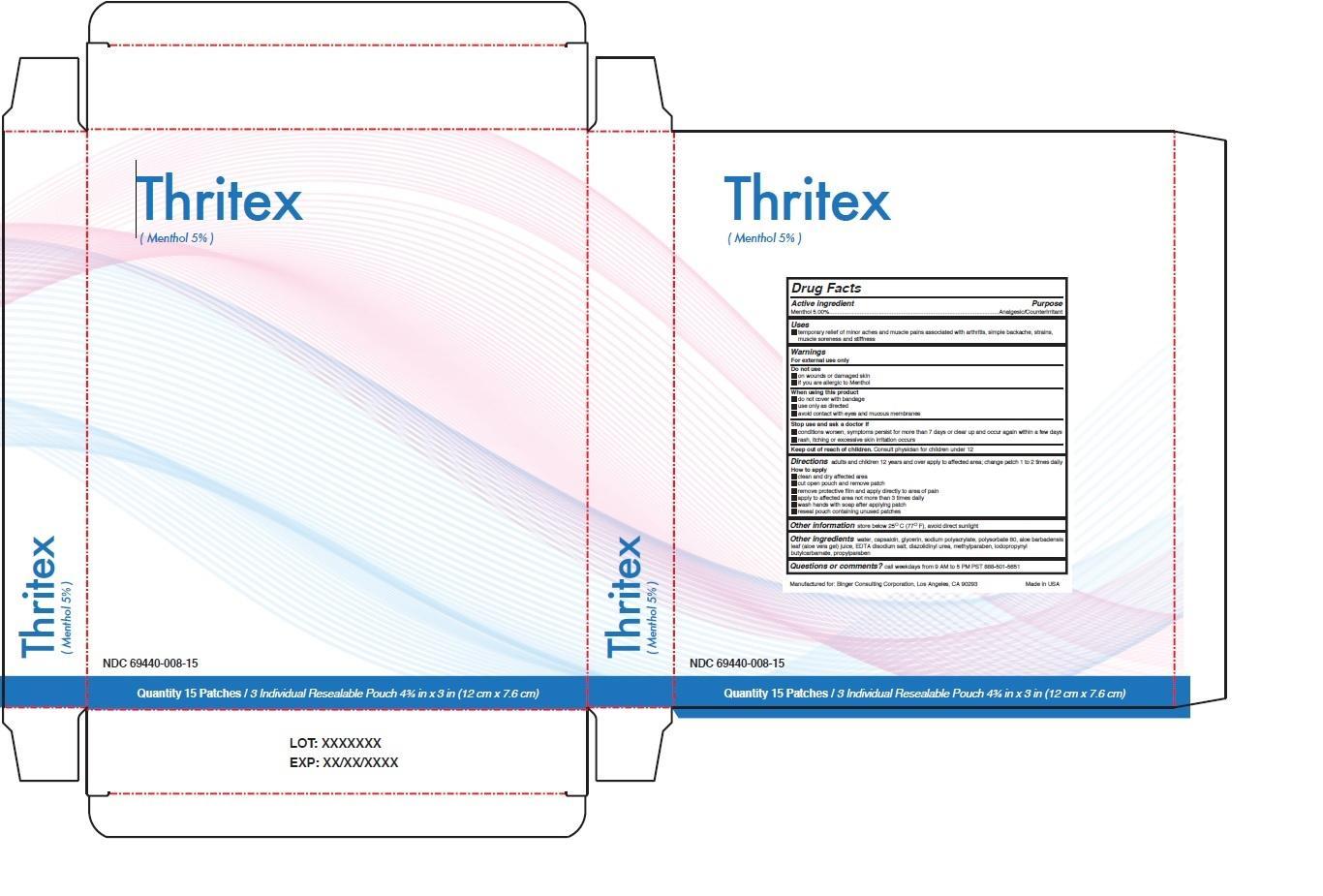
Label: THRITEX- menthol patch
- NDC Code(s): 69440-008-15
- Packager: Binger Consulting Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 11, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- KEEP OUT OF REACH OF CHILDREN
- Uses
-
Warnings
For external use only
Do not use
• on wounds or damaged skin
• if you are allergic to Menthol
When using this product
• do not cover with bandage
• use only as directed
• avoid contact with eyes and mucous membranes
Stop use and ask a doctor if
• conditions worsen, symptoms persist for more than 7 days or clear up and occur again within a few days
• rash, itching or excessive skin irritation occurs
-
DOSAGE & ADMINISTRATION
Directions adults and children 12 years and over apply to affected area; change patch 1 to 2 times daily
How to apply
• clean and dry affected area
• cut open pouch and remove patch
• remove protective film and apply directly to area of pain
• apply to affected area not more than 3 times daily
• wash hands with soap after applying patch
• reseal pouch containing unused patches.
- INACTIVE INGREDIENT
- QUESTIONS
- STORAGE AND HANDLING
- Packaging
-
INGREDIENTS AND APPEARANCE
THRITEX
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69440-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 5 g in 100 g Inactive Ingredients Ingredient Name Strength CAPSAICIN (UNII: S07O44R1ZM) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ALOE VERA LEAF (UNII: ZY81Z83H0X) EDETATE DISODIUM (UNII: 7FLD91C86K) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69440-008-15 15 in 1 BOX 01/01/2015 1 100 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/01/2015 Labeler - Binger Consulting Corporation (079635976) Establishment Name Address ID/FEI Business Operations Active Intelligence, LLC 080416593 manufacture(69440-008)