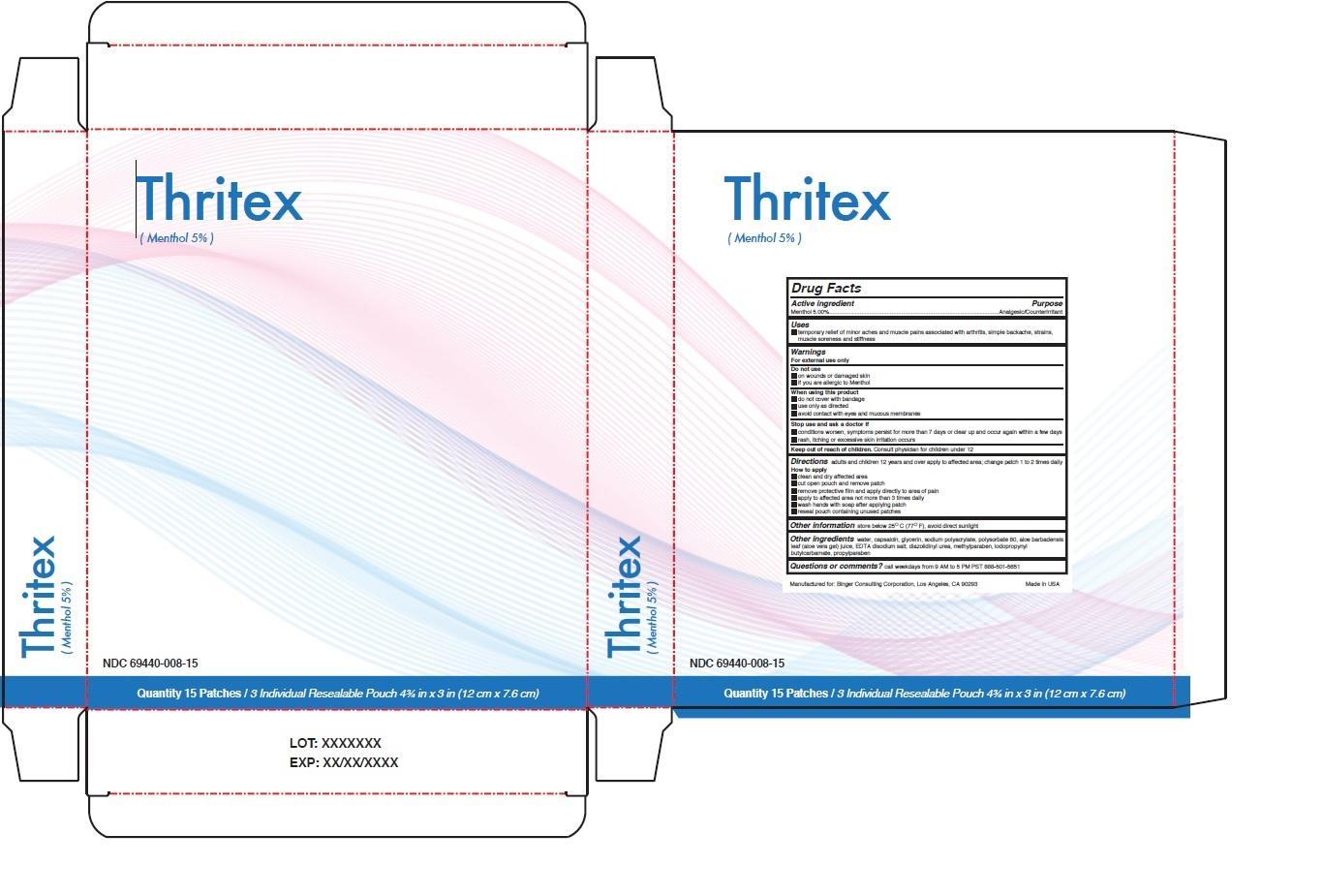
Uses
• temporary relief of minor aches and muscle pains associated with arthritis, simple backache, strains, muscle soreness and stiffness
Warnings
For external use only
Do not use
• on wounds or damaged skin
• if you are allergic to Menthol
When using this product
• do not cover with bandage
• use only as directed
• avoid contact with eyes and mucous membranes
Stop use and ask a doctor if
• conditions worsen, symptoms persist for more than 7 days or clear up and occur again within a few days
• rash, itching or excessive skin irritation occurs
Directions adults and children 12 years and over apply to affected area; change patch 1 to 2 times daily
How to apply
• clean and dry affected area
• cut open pouch and remove patch
• remove protective film and apply directly to area of pain
• apply to affected area not more than 3 times daily
• wash hands with soap after applying patch
• reseal pouch containing unused patches.