Label: CONTROL DANDRUFF THERAPY- dandruff therapy shampoo shampoo
- NDC Code(s): 50718-0021-1, 50718-0021-2
- Packager: Kamedis
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
When using this product
- avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water
Stop use and ask a doctor if
- condition worsens or does not improve after regular use of this product as directed
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
- If condition covers a large area of the body, consult your doctor before using this product.
- SPL UNCLASSIFIED SECTION
-
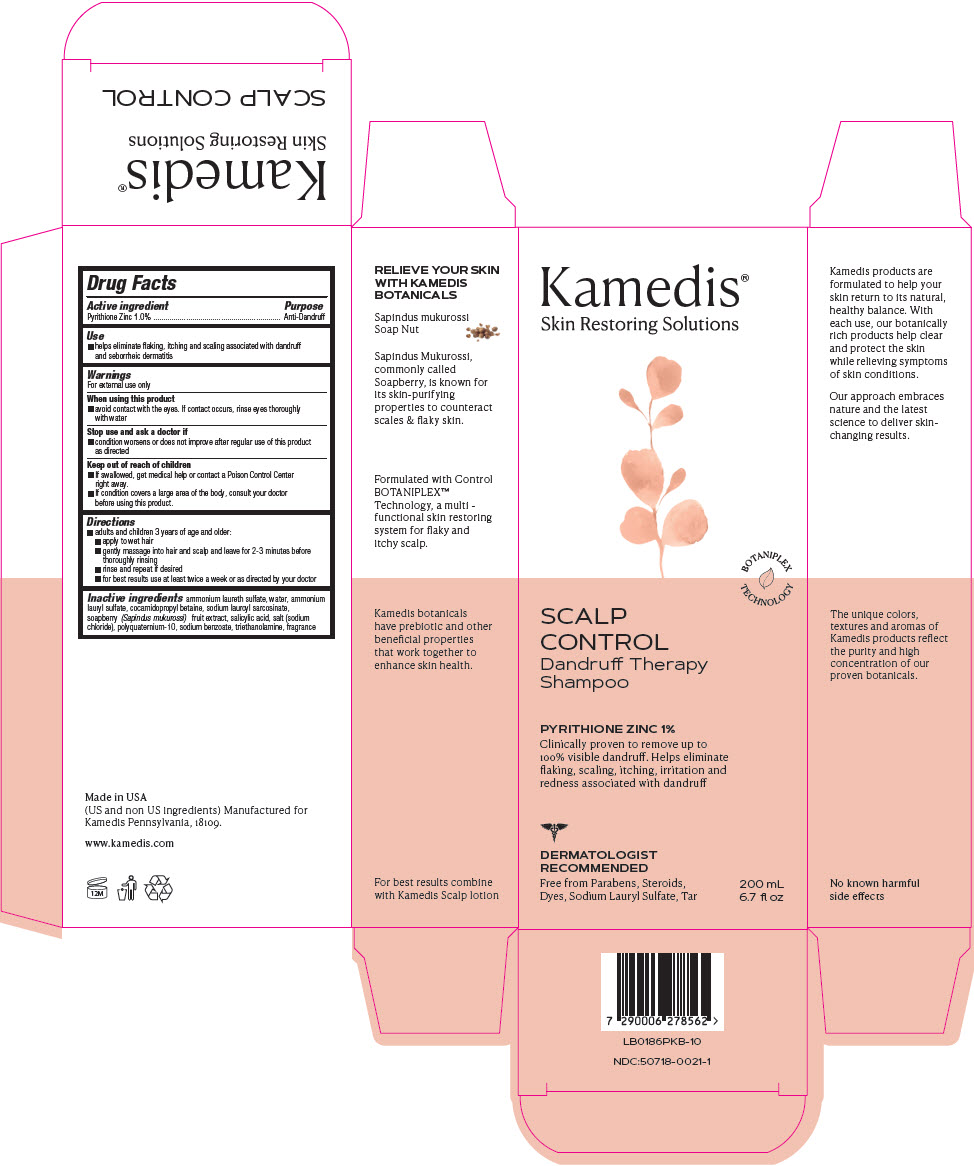
PRINCIPAL DISPLAY PANEL - 200 mL Bottle Carton
Kamedis®
Skin Restoring SolutionsBOTANIPLEX
TECHNOLOGYSCALP
CONTROL
Dandruff Therapy
ShampooPYRITHIONE ZINC 1%
Clinically proven to remove up to
100% visible dandruff. Helps eliminate
flaking, scaling, itching, irritation and
redness associated with dandruffDERMATOLOGIST
RECOMMENDEDFree from Parabens, Steroids,
Dyes, Sodium Lauryl Sulfate, Tar200 mL
6.7 fl oz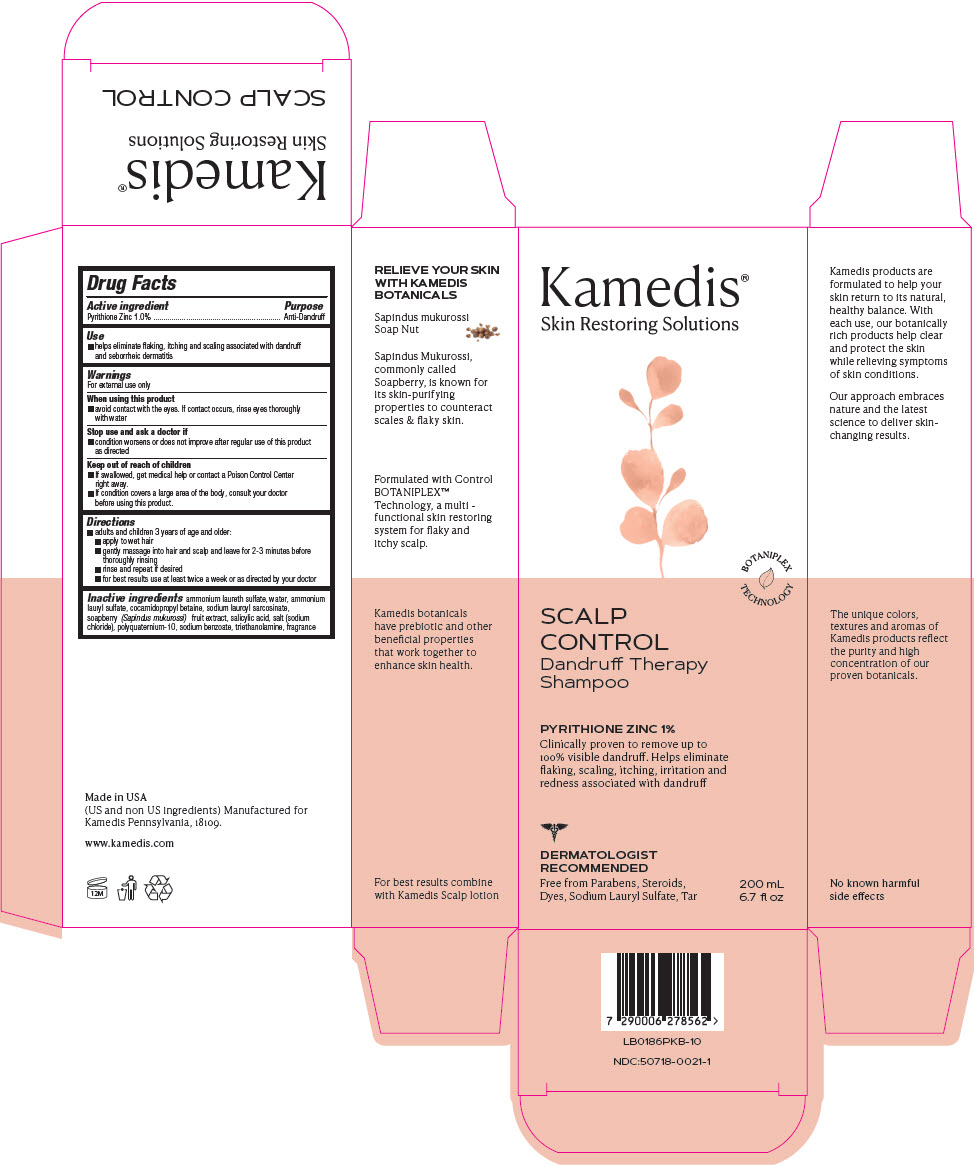
-
INGREDIENTS AND APPEARANCE
CONTROL DANDRUFF THERAPY
dandruff therapy shampoo shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50718-0021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SALICYLIC ACID (UNII: O414PZ4LPZ) SODIUM CHLORIDE (UNII: 451W47IQ8X) SAPINDUS MUKOROSSI FRUIT (UNII: 66H9NW427Y) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) AMMONIUM LAURETH-2 SULFATE (UNII: 698O4Z48G6) POLYQUATERNIUM-10 (10000 MPA.S AT 2%) (UNII: PI1STR9QYH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50718-0021-1 1 in 1 CARTON 01/01/2018 1 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:50718-0021-2 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M032 01/01/2018 Labeler - Kamedis (080311300)