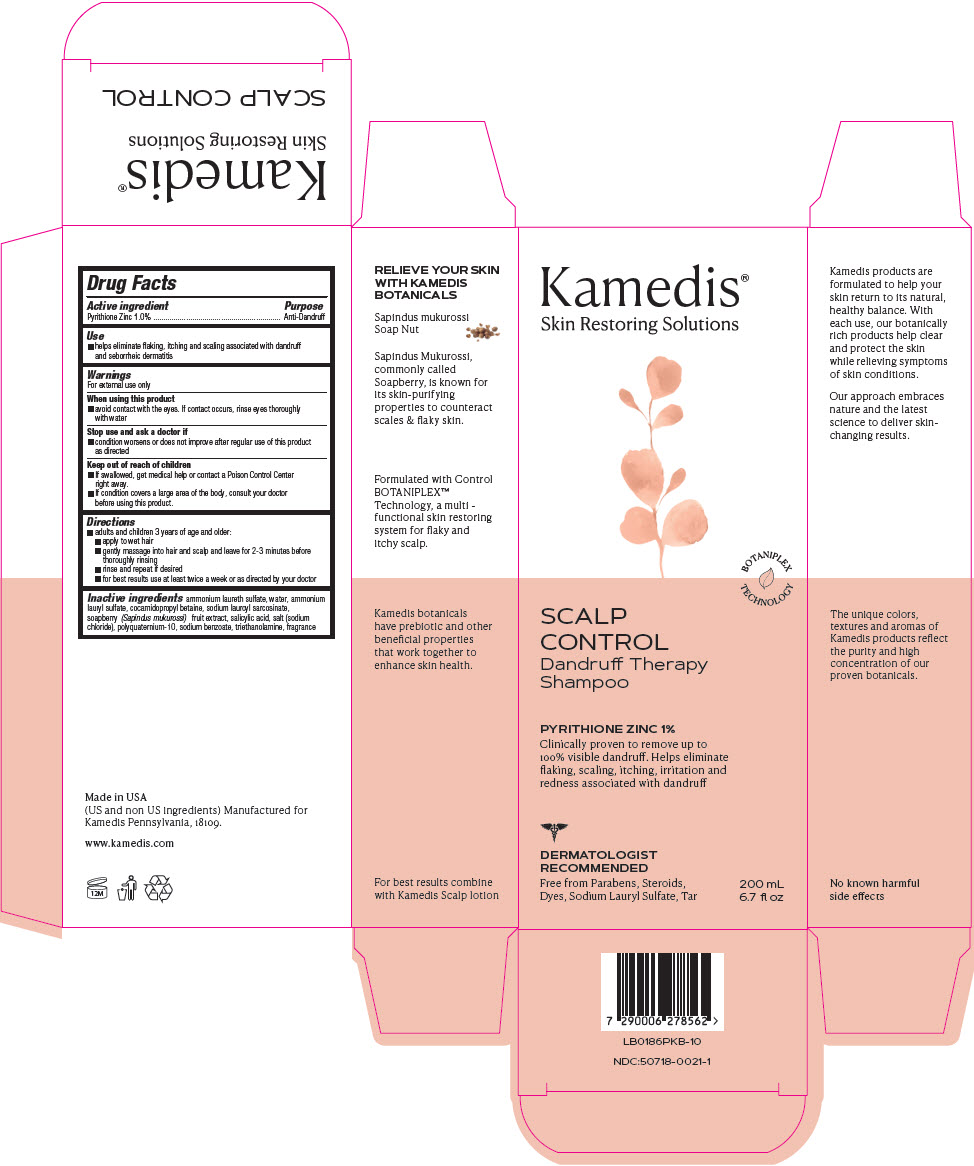
Drug Facts
When using this product
- avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water
Stop use and ask a doctor if
- condition worsens or does not improve after regular use of this product as directed
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
- If condition covers a large area of the body, consult your doctor before using this product.
PRINCIPAL DISPLAY PANEL - 200 mL Bottle Carton
Kamedis®
Skin Restoring Solutions
BOTANIPLEX
TECHNOLOGY
SCALP
CONTROL
Dandruff Therapy
Shampoo
PYRITHIONE ZINC 1%
Clinically proven to remove up to
100% visible dandruff. Helps eliminate
flaking, scaling, itching, irritation and
redness associated with dandruff
DERMATOLOGIST
RECOMMENDED
Free from Parabens, Steroids,
Dyes, Sodium Lauryl Sulfate, Tar
200 mL
6.7 fl oz